Human articular chondrocytes express ChemR23 and chemerin; ChemR23 promotes inflammatory signalling upon binding the ligand chemerin(21-157)
- PMID: 21192818
- PMCID: PMC3046541
- DOI: 10.1186/ar3215
Human articular chondrocytes express ChemR23 and chemerin; ChemR23 promotes inflammatory signalling upon binding the ligand chemerin(21-157)
Abstract
Introduction: Chemerin is a chemotactic peptide which directs leukocytes expressing the chemokine-like receptor ChemR23 towards sites of inflammation. ChemR23 is a G protein-coupled receptor which binds several different ligands, and it is also expressed by other cell types such as adipocytes. In addition to chemotaxis, recent reports suggest that ChemR23 is capable of mediating either inflammatory or anti-inflammatory effects, depending on the type of ligand it binds. In the present study, we aimed to clarify whether human chondrocytes express ChemR23 and chemerin, and whether chemerin/ChemR23 signalling could affect secretion of inflammatory mediators.
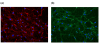
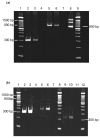
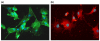
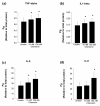
Methods: Tissue sections were taken from human knee joints and labelled with antibodies towards chemerin and ChemR23. Chondrocytes from cartilage tissue were isolated, cultured and assessed for chemerin and ChemR23 expression by PCR and immunolabelling. Receptor activation and intracellular signalling were studied by assessment of phosphorylated mitogen activated protein kinases (MAPKs) and phosphorylated Akt after stimulating cells with recombinant chemerin(21-157). Biological effects of chemerin(21-157) were investigated by measuring secretion of pro-inflammatory cytokines and metalloproteases in cell supernatants.
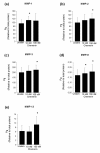
Results: Both serially cultured human articular chondrocytes and resident cells in native cartilage expressed chemerin and ChemR23. Stimulating cells with chemerin(21-157) resulted in phosphorylation of p44/p42 MAPKs (ERK 1/2) and Akt (Ser 473). Also, significantly enhanced levels of the pro-inflammatory cytokines interleukin-6 (IL-6), interleukin-8 (IL-8), tumour necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), and the matrix metalloproteases MMP-1, MMP-2, MMP-3, MMP-8 and MMP-13 were detected.
Conclusions: These results demonstrate that human chondrocytes express both the receptor ChemR23 and the ligand chemerin. Chemerin(21-157) stimulation engaged signal-transduction pathways that further promoted inflammatory signalling in chondrocytes, as judged by an enhanced secretion of cytokines and metalloproteases. Taken together, the previously reported chemotaxis and the present findings suggest that the receptor and its ligand may play pivotal roles in joint inflammation.
Figures



Comment in
-
Chemerin/ChemR23 pathway: a system beyond chemokines.Arthritis Res Ther. 2011 Apr 6;13(2):104. doi: 10.1186/ar3273. Arthritis Res Ther. 2011. PMID: 21542878 Free PMC article.
References
-
- Wittamer V, Bondue B, Guillabert A, Vassart G, Parmentier M, Communi D. Neutrophil-mediated maturation of chemerin: a link between innate and adaptive immunity. J Immunol. 2005;175:487–493. - PubMed
-
- Albanesi C, Scarponi C, Pallotta S, Daniele R, Bosisio D, Madonna S, Fortugno P, Gonzalvo-Feo S, Franssen JD, Parmentier M, De Pita O, Girolomoni G, Sozzani S. Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment. J Exp Med. 2009;206:249–258. doi: 10.1084/jem.20080129. - DOI - PMC - PubMed
-
- Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, Brezillon S, Tyldesley R, Blanpain C, Detheux M, Mantovani A, Sozzani S, Vassart G, Parmentier M, Communi D. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198:977–985. doi: 10.1084/jem.20030382. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

