A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples
- PMID: 21192942
- PMCID: PMC3050993
- DOI: 10.1016/j.jim.2010.12.016
A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples
Erratum in
- J Immunol Methods. 2012 Feb 28;376(1-2):156
Abstract
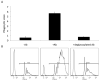
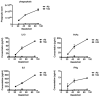
Phagocytosis can be induced via the engagement of Fcγ receptors by antibody-opsonized material. Furthermore, the efficiency of antibody-induced effector functions has been shown to be dramatically modulated by changes in antibody glycosylation. Because infection can modulate antibody glycans, which in turn modulate antibody functions, assays capable of determining the induction of effector functions rather than neutralization or titer provide a valuable opportunity to more fully characterize the quality of the adaptive immune response. Here we describe a robust and high-throughput flow cytometric assay to define the phagocytic activity of antigen-specific antibodies from clinical samples. This assay employs a monocytic cell line that expresses numerous Fc receptors: including inhibitory and activating, and high and low affinity receptors--allowing complex phenotypes to be studied. We demonstrate the adaptability of this high-throughput, flow-based assay to measure antigen-specific antibody-mediated phagocytosis against an array of viruses, including influenza, HIV, and dengue. The phagocytosis assay format further allows for simultaneous analysis of cytokine release, as well as determination of the role of specific Fcγ-receptor subtypes, making it a highly useful system for parsing differences in the ability of clinical and vaccine induced antibody samples to recruit this critical effector function.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures




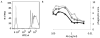


References
-
- Anthony RM, Ravetch JV. A novel role for the IgG Fc glycan: the anti-inflammatory activity of sialylated IgG Fcs. J Clin Immunol. 2010;30 1:S9–14. - PubMed
-
- Aslam A, Quinn P, McIntosh RS, Shi J, Ghumra A, McKerrow JH, Bunting KA, Dunne DW, Doenhoff MJ, Morrison SL, Zhang K, Pleass RJ. Proteases from Schistosoma mansoni cercariae cleave IgE at solvent exposed interdomain regions. Mol Immunol. 2008;45:567–74. - PubMed
-
- Berasain P, Carmona C, Frangione B, Dalton JP, Goni F. Fasciola hepatica: parasite-secreted proteinases degrade all human IgG subclasses: determination of the specific cleavage sites and identification of the immunoglobulin fragments produced. Exp Parasitol. 2000;94:99–110. - PubMed
-
- Bernard J, Reveil B, Najman I, Liautaud-Roger F, Fouchard M, Picard O, Cattan A, Mabondzo A, Laverne S, Gallo RC, et al. Discriminating between protective and enhancing HIV antibodies. AIDS Res Hum Retroviruses. 1990;6:243–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

