Evaluation of the time resolved fluorescence immunoassay (TRFIA) for the detection of varicella zoster virus (VZV) antibodies following vaccination of healthcare workers
- PMID: 21192976
- PMCID: PMC5391041
- DOI: 10.1016/j.jviromet.2010.12.021
Evaluation of the time resolved fluorescence immunoassay (TRFIA) for the detection of varicella zoster virus (VZV) antibodies following vaccination of healthcare workers
Abstract
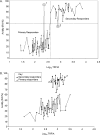
Determination of varicella zoster virus (VZV) immunity in healthcare workers without a history of chickenpox is important for identifying those in need of vOka vaccination. Post immunisation, healthcare workers in the UK who work with high risk patients are tested for seroconversion. To assess the performance of the time-resolved fluorescence immunoassay (TRFIA) for the detection of antibody in vaccinated as well as unvaccinated individuals, a cut-off was first calculated. VZV-IgG specific avidity and titres six weeks after the first dose of vaccine were used to identify subjects with pre-existing immunity among a cohort of 110 healthcare workers. Those with high avidity (≥ 60%) were considered to have previous immunity to VZV and those with low or equivocal avidity (<60%) were considered naive. The former had antibody levels ≥ 400 mIU/mL and latter had levels < 400 mIU/mL. Comparison of the baseline values of the naive and immune groups allowed the estimation of a TRFIA cut-off value of > 130 mIU/mL which best discriminated between the two groups and this was confirmed by ROC analysis. Using this value, the sensitivity and specificity of TRFIA cut-off were 90% (95% CI 79-96), and 78% (95% CI 61-90) respectively in this population. A subset of samples tested by the gold standard Fluorescence Antibody to Membrane Antigen (FAMA) test showed 84% (54/64) agreement with TRFIA.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures



References
-
- Ampofo K, Saiman L, LaRussa PS, Steinberg SP, Annunziato P, Gershon AA. Persistence of immunity to live attenuated varicella vaccine in healthy adults. Clin Infect Dis. 2002;34:774–779. - PubMed
-
- Australian Technical Advisory Group on Immunisation (ATAGI)/National Health and Medical Research Council (NHMRC) The Australian Immunization Handbook. 8th. National Health & Medical Research Council; Canberra: 2003.
-
- Department of Health, U.K. Green Book (09.05.10) 2006 http://www.dh.gov.uk/en/Publichealth/Healthprotection/1mmunisation/Green....
-
- Gershon AA, LaRussa PS, Steinberg SP. Detection of antibodies to varicella-zoster virus using a latex agglutination assay. Clin Diagn Virol. 1994;2:271–277. - PubMed
-
- Gershon AA, Steinberg SP, LaRussa PS, Ferrara A, Hammerschlag M, Gelb L. Immunization of healthy adults with live attenuated varicella vaccine. J Infect Dis. 1988;158:132–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

