CXCR4 signaling mediates morphine-induced tactile hyperalgesia
- PMID: 21193025
- PMCID: PMC3039030
- DOI: 10.1016/j.bbi.2010.12.014
CXCR4 signaling mediates morphine-induced tactile hyperalgesia
Abstract
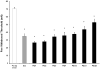
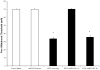
Morphine and related compounds are the first line of therapy in the treatment of moderate to severe pain. Over time, individuals taking opioids can develop an increasing sensitivity to noxious stimuli, even evolving into a painful response to previously non-noxious stimuli (opioid-induced hyperalgesia; OIH). The mechanism underlying OIH is not well understood although complex intracellular neural mechanisms, including opioid receptor desensitization and down-regulation, are believed to be major mechanisms underlying OIH. However, OIH may also be associated with changes in gene expression. A growing body of evidence suggests that cellular exposure to mu agonists upregulate chemokines/receptors and recent work from our laboratory implicates chemokine upregulation in a variety of neuropathic pain behaviors. Here we characterized the degree to which chemokines/receptors signaling is increased in primary afferent neurons of the dorsal root ganglion (DRG) following chronic morphine sulfate treatment and correlated these changes with tactile hyperalgesic behavior in rodents. We demonstrate that mRNA expression of the chemokine, stromal-derived factor-1 (SDF1/CXCL12) is upregulated following morphine treatment in sensory neurons of the rat. The release of SDF1 was found to be constitutive when compared with the activity dependent release of the C-C chemokine, monocyte chemoattractant protein-1 (MCP1/CCL2) in a line of F11 neuroblastoma-sensory neuron hybrid cells. We further determined that there is pronounced CXCR4 expression in satellite glial cells and following morphine treatment, increased functional CXCR4 expression in sensory neurons of the DRG. Moreover, intraperitoneal administration of the specific CXCR4 antagonist, AMD3100, completely reversed OIH in the rat. Taken together; the data suggest that opioid-induced SDF1/CXCR4 signaling is central to the development of long lasting OIH and that receptor antagonists represent a promising novel approach to the management of the side effects associated with the use of opioids for chronic pain management.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
SDF1-CXCR4 signaling contributes to persistent pain and hypersensitivity via regulating excitability of primary nociceptive neurons: involvement of ERK-dependent Nav1.8 up-regulation.J Neuroinflammation. 2015 Nov 24;12:219. doi: 10.1186/s12974-015-0441-2. J Neuroinflammation. 2015. PMID: 26597700 Free PMC article.
-
Increased chemokine signaling in a model of HIV1-associated peripheral neuropathy.Mol Pain. 2009 Aug 12;5:48. doi: 10.1186/1744-8069-5-48. Mol Pain. 2009. PMID: 19674450 Free PMC article.
-
CXCR4 chemokine receptor signaling mediates pain hypersensitivity in association with antiretroviral toxic neuropathy.Brain Behav Immun. 2007 Jul;21(5):581-91. doi: 10.1016/j.bbi.2006.12.003. Epub 2007 Feb 9. Brain Behav Immun. 2007. PMID: 17292584 Free PMC article.
-
Opioid-induced hyperalgesia: Cellular and molecular mechanisms.Neuroscience. 2016 Dec 3;338:160-182. doi: 10.1016/j.neuroscience.2016.06.029. Epub 2016 Jun 23. Neuroscience. 2016. PMID: 27346146 Review.
-
Chemokines as pain mediators and modulators.Curr Opin Anaesthesiol. 2008 Oct;21(5):580-5. doi: 10.1097/ACO.0b013e32830eb69d. Curr Opin Anaesthesiol. 2008. PMID: 18784482 Free PMC article. Review.
Cited by
-
Opioid-induced central immune signaling: implications for opioid analgesia.Headache. 2015 Apr;55(4):475-89. doi: 10.1111/head.12552. Epub 2015 Mar 31. Headache. 2015. PMID: 25833219 Free PMC article. Review.
-
Acute morphine activates satellite glial cells and up-regulates IL-1β in dorsal root ganglia in mice via matrix metalloprotease-9.Mol Pain. 2012 Mar 22;8:18. doi: 10.1186/1744-8069-8-18. Mol Pain. 2012. PMID: 22439811 Free PMC article.
-
Functions of the chemokine receptor CXCR4 in the central nervous system and its regulation by μ-opioid receptors.Int Rev Neurobiol. 2014;118:105-28. doi: 10.1016/B978-0-12-801284-0.00005-1. Int Rev Neurobiol. 2014. PMID: 25175863 Free PMC article. Review.
-
SDF1-CXCR4 signaling contributes to persistent pain and hypersensitivity via regulating excitability of primary nociceptive neurons: involvement of ERK-dependent Nav1.8 up-regulation.J Neuroinflammation. 2015 Nov 24;12:219. doi: 10.1186/s12974-015-0441-2. J Neuroinflammation. 2015. PMID: 26597700 Free PMC article.
-
A Randomized, Open-Label Phase 2 Study of the CXCR4 Inhibitor LY2510924 in Combination with Sunitinib Versus Sunitinib Alone in Patients with Metastatic Renal Cell Carcinoma (RCC).Target Oncol. 2016 Oct;11(5):643-653. doi: 10.1007/s11523-016-0434-9. Target Oncol. 2016. PMID: 27154357 Clinical Trial.
References
-
- Angst MS, Koppert W, Pahl I, Clark DJ, Schmelz M. Short-term infusion of the mu-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. Pain. 2003;106:49–57. - PubMed
-
- Arner S, Rawal N, Gustafsson LL. Clinical experience of long-term treatment with epidural and intrathecal opioids--a nationwide survey. Acta Anaesthesiol Scand. 1988;32:253–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

