A pilot trial of the microtubule-interacting peptide (NAP) in mice overexpressing alpha-synuclein shows improvement in motor function and reduction of alpha-synuclein inclusions
- PMID: 21193046
- PMCID: PMC3046337
- DOI: 10.1016/j.mcn.2010.12.011
A pilot trial of the microtubule-interacting peptide (NAP) in mice overexpressing alpha-synuclein shows improvement in motor function and reduction of alpha-synuclein inclusions
Abstract
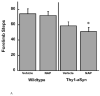
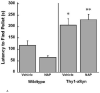
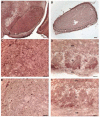
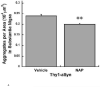
Abnormal accumulation of α-synuclein is associated with several neurodegenerative disorders (synucleinopathies), including sporadic Parkinson's disease (PD). Genetic mutations and multiplication of α-synuclein cause familial forms of PD and polymorphisms in the α-synuclein gene are associated with PD risk. Overexpression of α-synuclein can impair essential functions within the cell such as microtubule-dependent transport, suggesting that compounds that act on the microtubule system may have therapeutic benefit for synucleinopathies. In this study, mice overexpressing human wildtype α-synuclein under the Thy1 promoter (Thy1-aSyn) and littermate wildtype control mice were administered daily the microtubule-interacting peptide NAPVSIPQ (NAP; also known as davunetide or AL-108) intranasally for 2 months starting at 1 month of age, in a regimen known to produce effective concentrations of the peptide in mouse brain. Motor performance, coordination, and activity were assessed at the end of treatment. Olfactory function, which is altered in PD, was measured 1 month later. Mice were sacrificed at 4.5 months of age, and their brains examined for proteinase K-resistant α-synuclein inclusions in the substantia nigra and olfactory bulb. NAP-treated Thy1-aSyn mice showed a 38% decrease in the number of errors per step in the challenging beam traversal test and a reduction in proteinase K-resistant α-synuclein inclusions in the substantia nigra compared to vehicle treated transgenics. The data indicate a significant behavioral benefit and a long lasting improvement of α-synuclein pathology following administration of a short term (2 months) NAP administration in a mouse model of synucleinopathy.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures












Similar articles
-
Olfactory deficits in mice overexpressing human wildtype alpha-synuclein.Eur J Neurosci. 2008 Jul;28(2):247-56. doi: 10.1111/j.1460-9568.2008.06346.x. Eur J Neurosci. 2008. PMID: 18702696 Free PMC article.
-
A Molecular Tweezer Ameliorates Motor Deficits in Mice Overexpressing α-Synuclein.Neurotherapeutics. 2017 Oct;14(4):1107-1119. doi: 10.1007/s13311-017-0544-9. Neurotherapeutics. 2017. PMID: 28585223 Free PMC article.
-
α-Synuclein BAC transgenic mice exhibit RBD-like behaviour and hyposmia: a prodromal Parkinson's disease model.Brain. 2020 Jan 1;143(1):249-265. doi: 10.1093/brain/awz380. Brain. 2020. PMID: 31816026
-
A Mouse Model to Test Novel Therapeutics for Parkinson's Disease: an Update on the Thy1-aSyn ("line 61") Mice.Neurotherapeutics. 2023 Jan;20(1):97-116. doi: 10.1007/s13311-022-01338-0. Epub 2023 Jan 30. Neurotherapeutics. 2023. PMID: 36715870 Free PMC article. Review.
-
Strengths and limitations of genetic mouse models of Parkinson's disease.Parkinsonism Relat Disord. 2008;14 Suppl 2(Suppl 2):S84-7. doi: 10.1016/j.parkreldis.2008.04.004. Epub 2008 Jun 27. Parkinsonism Relat Disord. 2008. PMID: 18585084 Free PMC article. Review.
Cited by
-
Pharmacological inhibition of CSF1R by GW2580 reduces microglial proliferation and is protective against neuroinflammation and dopaminergic neurodegeneration.FASEB J. 2020 Jan;34(1):1679-1694. doi: 10.1096/fj.201900567RR. Epub 2019 Dec 4. FASEB J. 2020. PMID: 31914683 Free PMC article.
-
Cognitive deficits in a mouse model of pre-manifest Parkinson's disease.Eur J Neurosci. 2012 Mar;35(6):870-82. doi: 10.1111/j.1460-9568.2012.08012.x. Epub 2012 Feb 22. Eur J Neurosci. 2012. PMID: 22356593 Free PMC article.
-
How Well Do Rodent Models of Parkinson's Disease Recapitulate Early Non-Motor Phenotypes? A Systematic Review.Biomedicines. 2022 Nov 24;10(12):3026. doi: 10.3390/biomedicines10123026. Biomedicines. 2022. PMID: 36551782 Free PMC article. Review.
-
β-III Tubulin fragments inhibit α-synuclein accumulation in models of multiple system atrophy.J Biol Chem. 2014 Aug 29;289(35):24374-82. doi: 10.1074/jbc.M114.557215. Epub 2014 Jul 15. J Biol Chem. 2014. PMID: 25028513 Free PMC article.
-
Sex-Specific ADNP/NAP (Davunetide) Regulation of Cocaine-Induced Plasticity.J Mol Neurosci. 2024 Sep 10;74(3):76. doi: 10.1007/s12031-024-02234-2. J Mol Neurosci. 2024. PMID: 39251453 Free PMC article.
References
-
- Alcalay RN, Giladi E, Pick CG, Gozes I. Intranasal administration of NAP, a neuroprotective peptide, decreases anxiety-like behavior in aging mice in the elevated plus maze. Neurosci. Lett. 2004;361:128–131. - PubMed
-
- Alim MA, Hossain MS, Arima K, Takeda K, Izumiyama Y, Nakamura M, Kaji H, Shinoda T, Hisanaga S, Ueda K. Tubulin seeds alpha-synuclein fibril formation. J. Biol. Chem. 2002;277(3):2112–2117. - PubMed
-
- Ashur-Fabian O, Segal-Ruder Y, Skutelsky E, Brenneman DE, Steingart RA, Giladi E, Gozes I. The neuroprotective peptide NAP inhibits the aggregation of the beta-amyloid peptide. Peptides. 2003;24:1413–1423. - PubMed
-
- Bassan M, Zamostiano R, Davidson A, Pinhasov A, Giladi E, Perl O, Bassan H, Blat C, Gibney, Glazner G, Brenneman DE, Gozes I. Complete sequence of a novel protein containing a femtomolar-activity-dependent neuroprotective peptide. J. Neurochem. 1999;72(3):1283–1293. - PubMed
-
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24:197–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

