Establishment of a xenograft model of human myelodysplastic syndromes
- PMID: 21193418
- PMCID: PMC3069231
- DOI: 10.3324/haematol.2010.027557
Establishment of a xenograft model of human myelodysplastic syndromes
Abstract
Background: To understand how myelodysplastic syndrome cells evolve from normal stem cells and gain competitive advantages over normal hematopoiesis, we established a murine xenograft model harboring bone marrow cells from patients with myelodysplastic syndromes or acute myeloid leukemia with myelodysplasia-related changes.
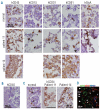
Design and methods: Bone marrow CD34(+) cells obtained from patients were injected, with or without human mesenchymal stem cells, into the bone marrow of non-obese diabetic/severe combined immunodeficient/IL2Rγ(null) hosts. Engraftment and differentiation of cells derived from the patients were investigated by flow cytometry and immunohistochemical analysis.
Results: Co-injection of patients' cells and human mesenchymal stem cells led to successful engraftment of patient-derived cells that maintained the immunophenotypes and genomic abnormalities of the original patients. Myelodysplastic syndrome-originated clones differentiated into mature neutrophils, megakaryocytes, and erythroblasts. Two of the samples derived from patients with acute myeloid leukemia with myelodysplasia-related changes were able to sustain neoplastic growth into the next generation while these cells had limited differentiation ability in the murine host. The hematopoiesis of mice engrafted with patients' cells was significantly suppressed even when human cells accounted for less than 1% of total marrow mononuclear cells. Histological studies revealed invasion of the endosteal surface by patient-derived CD34(+) cells and disruption of extracellular matrix architecture, which probably caused inhibition of murine hematopoiesis.
Conclusions: We established murine models of human myelodysplastic syndromes using cells obtained from patients: the presence of neoplastic cells was associated with the suppression of normal host hematopoiesis. The efficiency of engraftment was related to the presence of an abnormality in chromosome 7.
Figures


References
-
- Steensma DP, Tefferi A. The myelodysplastic syndrome(s): a perspective and review highlighting current controversies. Leuk Res. 2003;27(2):95–120. - PubMed
-
- Nimer SD. Myelodysplastic syndromes. Blood. 2008;111 (10):4841–51. - PubMed
-
- Heaney ML, Golde DW. Myelodysplasia. N Engl J Med. 1999;340(21):1649–60. - PubMed
-
- Haase D, Fonatsch C, Freund M, Wormann B, Bodenstein H, Bartels H, et al. Cytogenetic findings in 179 patients with myelodysplastic syndromes. Ann Hematol. 1995;70(4):171–87. - PubMed
-
- Parlier V, van Melle G, Beris P, Schmidt PM, Tobler A, Haller E, et al. Hematologic, clinical, and cytogenetic analysis in 109 patients with primary myelodysplastic syndrome. Prognostic significance of morphology and chromosome findings. Cancer Genet Cytogenet. 1994;78(2):219–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

