PERK activation at low glucose concentration is mediated by SERCA pump inhibition and confers preemptive cytoprotection to pancreatic β-cells
- PMID: 21193559
- PMCID: PMC3070211
- DOI: 10.1210/me.2010-0309
PERK activation at low glucose concentration is mediated by SERCA pump inhibition and confers preemptive cytoprotection to pancreatic β-cells
Abstract
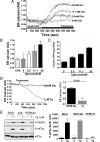
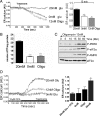
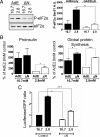
Protein kinase R-like ER kinase (PERK) is activated at physiologically low glucose concentrations in pancreatic β-cells. However, the molecular mechanisms by which PERK is activated under these conditions and its role in β-cell function are poorly understood. In this report, we investigated, in dispersed rat islets of Langerhans and mouse insulinoma-6 (MIN6) cells, the relationship between extracellular glucose concentration, the free endoplasmic reticulum (ER) calcium concentration ([Ca(2+)](ER)) measured directly using an ER targeted fluorescence resonance energy transfer-based calcium sensor, and the activation of PERK. We found that a decrease in glucose concentration leads to a concentration-dependent reduction in [Ca(2+)](ER) that parallels the activation of PERK and the phosphorylation of its substrate eukaryotic initiation factor-2α. We provide evidence that this decrease in [Ca(2+)](ER) is caused by a decrease in sarcoplasmic/ER Ca(2+)-ATPase pump activity mediated by a reduction in the energy status of the cell. Importantly, we also report that PERK-dependent eukaryotic initiation factor-2α phosphorylation at low glucose concentration plays a significant role in 1) the regulation of both proinsulin and global protein synthesis, 2) cell viability, and 3) conferring preemptive cytoprotection against ER stress. Taken together, these results provide evidence that a decrease in the ATP/energy status of the cell in response to a decrease in glucose concentration results in sarcoplasmic/ER Ca(2+)-ATPase pump inhibition, the efflux of Ca(2+) from the ER, and the activation of PERK, which plays an important role in both pancreatic β-cell function and survival.
Figures
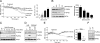


References
-
- Gomez E, Powell ML, Greenman IC, Herbert TP. 2004. Glucose-stimulated protein synthesis in pancreatic β-cells parallels an increase in the availability of the translational ternary complex (eIF2-GTP. Met-tRNAi) and the dephosphorylation of eIF2α. J Biol Chem 279:53937–53946 - PubMed
-
- Itoh N, Okamoto H. 1980. Translational control of proinsulin synthesis by glucose. Nature 283:100–102 - PubMed
-
- Itoh N, Sei T, Nose K, Okamoto H. 1978. Glucose stimulation of the proinsulin synthesis in isolated pancreatic islets without increasing amount of proinsulin mRNA. FEBS Lett 93:343–347 - PubMed
-
- Permutt MA. 1974. Effect of glucose on initiation and elongation rates in isolated rat pancreatic islets. J Biol Chem 249:2738–2742 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

