Real-time solvent tolerance analysis of pseudomonas sp. strain VLB120{Delta}C catalytic biofilms
- PMID: 21193676
- PMCID: PMC3067263
- DOI: 10.1128/AEM.02498-10
Real-time solvent tolerance analysis of pseudomonas sp. strain VLB120{Delta}C catalytic biofilms
Abstract
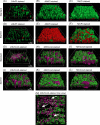
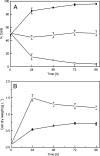
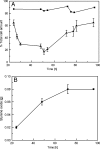
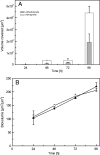
Biofilms are ubiquitous surface-associated microbial communities embedded in an extracellular polymeric (EPS) matrix, which gives the biofilm structural integrity and strength. It is often reported that biofilm-grown cells exhibit enhanced tolerance toward adverse environmental stress conditions, and thus there has been a growing interest in recent years to use biofilms for biotechnological applications. We present a time- and locus-resolved, noninvasive, quantitative approach to study biofilm development and its response to the toxic solvent styrene. Pseudomonas sp. strain VLB120ΔC-BT-gfp1 was grown in modified flow-cell reactors and exposed to the solvent styrene. Biofilm-grown cells displayed stable catalytic activity, producing (S)-styrene oxide continuously during the experimental period. The pillar-like structure and growth rate of the biofilm was not influenced by the presence of the solvent. However, the cells experience severe membrane damage during styrene treatment, although they obviously are able to adapt to the solvent, as the amount of permeabilized cells decreased from 75 to 80% down to 40% in 48 h. Concomitantly, the fraction of concanavalin A (ConA)-stainable EPS increased, substantiating the assumption that those polysaccharides play a major role in structural integrity and enhanced biofilm tolerance toward toxic environments. Compared to control experiments with planktonic grown cells, the Pseudomonas biofilm adapted much better to toxic concentrations of styrene, as nearly 65% of biofilm cells were not permeabilized (viable), compared to only 7% in analogous planktonic cultures. These findings underline the robustness of biofilms under stress conditions and its potential for fine chemical syntheses.
Figures

Similar articles
-
Segmented flow is controlling growth of catalytic biofilms in continuous multiphase microreactors.Biotechnol Bioeng. 2014 Sep;111(9):1831-40. doi: 10.1002/bit.25256. Epub 2014 Jun 16. Biotechnol Bioeng. 2014. PMID: 24729096
-
Engineering of Pseudomonas taiwanensis VLB120 for constitutive solvent tolerance and increased specific styrene epoxidation activity.Appl Environ Microbiol. 2014 Oct;80(20):6539-48. doi: 10.1128/AEM.01940-14. Epub 2014 Aug 15. Appl Environ Microbiol. 2014. PMID: 25128338 Free PMC article.
-
Carbon metabolism and product inhibition determine the epoxidation efficiency of solvent-tolerant Pseudomonas sp. strain VLB120DeltaC.Biotechnol Bioeng. 2007 Dec 15;98(6):1219-29. doi: 10.1002/bit.21496. Biotechnol Bioeng. 2007. PMID: 17514751
-
Mechanisms of biofilm resistance to antimicrobial agents.Trends Microbiol. 2001 Jan;9(1):34-9. doi: 10.1016/s0966-842x(00)01913-2. Trends Microbiol. 2001. PMID: 11166241 Review.
-
What's on the Outside Matters: The Role of the Extracellular Polymeric Substance of Gram-negative Biofilms in Evading Host Immunity and as a Target for Therapeutic Intervention.J Biol Chem. 2016 Jun 10;291(24):12538-12546. doi: 10.1074/jbc.R115.707547. Epub 2016 Apr 21. J Biol Chem. 2016. PMID: 27129225 Free PMC article. Review.
Cited by
-
Production host selection for asymmetric styrene epoxidation: Escherichia coli vs. solvent-tolerant Pseudomonas.J Ind Microbiol Biotechnol. 2012 Aug;39(8):1125-33. doi: 10.1007/s10295-012-1126-9. Epub 2012 Apr 17. J Ind Microbiol Biotechnol. 2012. PMID: 22526330
-
Rapid enzyme regeneration results in the striking catalytic longevity of an engineered, single species, biocatalytic biofilm.Microb Cell Fact. 2016 Oct 21;15(1):180. doi: 10.1186/s12934-016-0579-3. Microb Cell Fact. 2016. PMID: 27769259 Free PMC article.
-
Development of Flow State Self-Regulation Skills and Coping With Musical Performance Anxiety: Design and Evaluation of an Electronically Implemented Psychological Program.Front Psychol. 2022 Jun 17;13:899621. doi: 10.3389/fpsyg.2022.899621. eCollection 2022. Front Psychol. 2022. PMID: 35783805 Free PMC article.
-
Optimisation of engineered Escherichia coli biofilms for enzymatic biosynthesis of l-halotryptophans.AMB Express. 2013 Nov 4;3(1):66. doi: 10.1186/2191-0855-3-66. AMB Express. 2013. PMID: 24188712 Free PMC article.
-
Growth of Pseudomonas taiwanensis VLB120∆C biofilms in the presence of n-butanol.Microb Biotechnol. 2017 Jul;10(4):745-755. doi: 10.1111/1751-7915.12413. Epub 2016 Oct 3. Microb Biotechnol. 2017. PMID: 27696696 Free PMC article.
References
-
- Bao, Y., D. P. Lies, H. Fu, and G. P. Roberts. 1991. An improved Tn7-based system for the single-copy insertion of cloned genes into chromosomes of gram-negative bacteria. Gene 109:167-168. - PubMed
-
- Branda, S. S., S. Vik, L. Friedman, and R. Kolter. 2005. Biofilms: the matrix revisited. Trends Microbiol. 13:20-26. - PubMed
-
- Brown, M. R., D. G. Allison, and P. Gilbert. 1988. Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? J. Antimicrob. Chemother. 22:777-780. - PubMed
-
- Chen, M. Y., D. J. Lee, Z. Yang, X. F. Peng, and J. Y. Lai. 2006. Fluorecent staining for study of extracellular polymeric substances in membrane biofouling layers. Environ. Sci. Technol. 40:6642-6646. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

