CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies
- PMID: 21193697
- PMCID: PMC3062409
- DOI: 10.1182/blood-2010-08-301945
CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies
Retraction in
-
Kohrt HE, Houot R, Goldstein MJ, Weiskopf K, Alizadeh AA, Brody J, Müller A, Pachynski R, Czerwinski D, Coutre S, Chao MP, Chen L, Tedder TF, Levy R. CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood. 2011;117(8):2423-2432.Blood. 2019 Aug 15;134(7):658. doi: 10.1182/blood.2019002416. Epub 2019 Jul 11. Blood. 2019. PMID: 31296533 Free PMC article. No abstract available.
Abstract
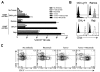
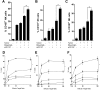
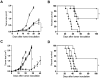
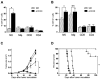
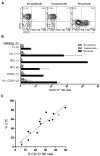
Antibody-dependent cell-mediated cytotoxicity (ADCC), which is largely mediated by natural killer (NK) cells, is thought to play an important role in the efficacy of rituximab, an anti-CD20 monoclonal antibody (mAb) used to treat patients with B-cell lymphomas. CD137 is a costimulatory molecule expressed on a variety of immune cells after activation, including NK cells. In the present study, we show that an anti-CD137 agonistic mAb enhances the antilymphoma activity of rituximab by enhancing ADCC. Human NK cells up-regulate CD137 after encountering rituximab-coated tumor B cells, and subsequent stimulation of these NK cells with anti-CD137 mAb enhances rituximab-dependent cytotoxicity against the lymphoma cells. In a syngeneic murine lymphoma model and in a xenotransplanted human lymphoma model, sequential administration of anti-CD20 mAb followed by anti-CD137 mAb had potent antilymphoma activity in vivo. These results support a novel, sequential antibody approach against B-cell malignancies by targeting first the tumor and then the host immune system.
Figures


References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. - PubMed
-
- Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. - PubMed
-
- Maloney DG, Grillo-Lopez AJ, Bodkin DJ, et al. IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin's lymphoma. J Clin Oncol. 1997;15(10):3266–3274. - PubMed
-
- Maloney DG, Grillo-Lopez AJ, White CA, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood. 1997;90(6):2188–2195. - PubMed
-
- Cartron G, Watier H, Golay J, Solal-Celigny P. From the bench to the bedside: ways to improve rituximab efficacy. Blood. 2004;104(9):2635–2642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

