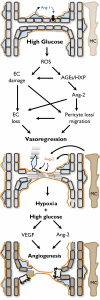
Diabetic retinopathy: targeting vasoregression
- PMID: 21193734
- PMCID: PMC3012202
- DOI: 10.2337/db10-0454
Diabetic retinopathy: targeting vasoregression
Figures




References
-
- Frank RN: Diabetic retinopathy. N Engl J Med 2004;350:48–58 - PubMed
-
- Gariano RF, Gardner TW: Retinal angiogenesis in development and disease. Nature 2005;438:960–966 - PubMed
-
- Duh E, Aiello LP: Vascular endothelial growth factor and diabetes: the agonist versus antagonist paradox. Diabetes 1999;48:1899–1906 - PubMed
-
- Carmeliet P: Angiogenesis in health and disease. Nat Med 2003;9:653–660 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

