Activation of vascular bone morphogenetic protein signaling in diabetes mellitus
- PMID: 21193740
- PMCID: PMC3042480
- DOI: 10.1161/CIRCRESAHA.110.236596
Activation of vascular bone morphogenetic protein signaling in diabetes mellitus
Abstract
Rationale: Diabetes mellitus is frequently complicated by cardiovascular disease, such as vascular calcification and endothelial dysfunction, which have been associated with bone morphogenetic proteins (BMPs).
Objective: To determine whether hyperglycemia in vitro and diabetes in vivo promote vascular BMP activity and correlate with vascular calcification.
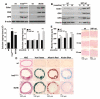
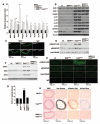
Methods and results: Increased glucose augmented expression of BMP-2 and BMP-4; the BMP inhibitors matrix Gla protein (MGP) and Noggin; activin-like kinase receptor (ALK)1, -2, -3 and -6; the BMP type 2 receptor; and the vascular endothelial growth factor in human aortic endothelial cells (HAECs). Diabetes induced expression of the same factors in the aortic wall of 3 animal models of diabetes, Ins2(Akita/+) mice, db/db mice, and HIP rats (rats transgenic for human islet amyloid polypeptide), representative of types 1 and 2 diabetes. Conditioned media from glucose-treated HAECs increased angiogenesis in bovine aortic endothelial cells, as mediated by BMP-4, and osteogenesis in calcifying vascular cells, as mediated by BMP-2. BMP-4, MGP, ALK1, and ALK2 were predominantly expressed on the endothelial side of the aorta, and small interfering RNA experiments showed that these genes were regulated as a group. Diabetic mice and rats showed a dramatic increase in aortic BMP activity, as demonstrated by SMAD1/5/8 phosphorylation. This was associated with increased osteogenesis and calcium accumulation. These changes were prevented in the Ins2(Akita/+) mice by breeding them with MGP transgenic mice, which increased aortic BMP inhibition.
Conclusions: Hyperglycemia and diabetes activate vascular BMP activity, which is instrumental in promoting vascular calcification and may be limited by increasing BMP inhibition.
Figures






References
-
- Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med. 1997;14(Suppl 5):S1–85. - PubMed
-
- Luscher TF, Creager MA, Beckman JA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part II. Circulation. 2003;108:1655–1661. - PubMed
-
- Wu KK, Huan Y. Diabetic atherosclerosis mouse models. Atherosclerosis. 2007;191:241–249. - PubMed
-
- Vattikuti R, Towler DA. Osteogenic regulation of vascular calcification: an early perspective. Am J Physiol Endocrinol Metab. 2004;286:E686–E696. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

