The slow cell death response when screening chemotherapeutic agents
- PMID: 21193989
- PMCID: PMC3210817
- DOI: 10.1007/s00280-010-1549-9
The slow cell death response when screening chemotherapeutic agents
Abstract
Purpose: To examine the correlation between cell death and a common surrogate of death used in screening assays, we compared cell death responses to those obtained with the sulforhodamine B (SRB) cell protein-based "cytotoxicity" assay.
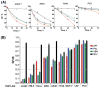
Method: With the SRB assay, the Hill equation was used to obtain an IC50 and final cell mass, or cell mass present at infinite agent concentrations, with eight adherent cell lines and four agents (32 agent/cell combinations). Cells were treated with high agent concentrations (well above the SRB IC50) and the death response determined as the time-dependent decrease in cells failing to bind both annexin V and vital fluorochromes by flow cytometry.
Results: Death kinetics were categorized as fast (5/32) (similar to the reference nonadherent Jurkat line), slow (17/32), or none (10/32), despite positive responses in the SRB assay in all cases. With slow cell death, a single exposure to a chemotherapeutic agent caused a slow, progressive increase in dead (necrotic) and dying (apoptotic) cells for at least 72 h.
Conclusions: Cell death (defined by annexin and/or fluorochrome binding) did not correlate with the standard SRB "cytotoxicity" assay. With the slow cell death response, a single exposure to an agent caused a slow conversion from vital to apoptotic and necrotic cells over at least 72 h (the longest time point examined). Here, increasing the time of exposure to agent concentrations modestly above the SRB IC50 provides a method of maximizing cell kill. If tumors respond similarly, sustained low doses of chemotherapeutic agents, rather than a log-kill, maximum tolerated dose strategy may be an optimal strategy of maximizing tumor cell death.
Conflict of interest statement
Figures



References
-
- Rubinstein LV, Shoemaker RH, Paull KD, Simon RM, Tosini S, Skehan P, Scudiero DA, Monks A, Boyd MR. Comparison of in vitro anticancer-drug-screening data generated with a tetrazolium assay versus a protein assay against a diverse panel of human tumor cell lines. J Natl Cancer Inst. 1990;82:1113–1118. - PubMed
-
- Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–1116. - PubMed
-
- Keepers YP, Pizao PE, Peters GJ, van Ark-Otte J, Winograd B, Pinedo HM. Comparison of the sulforhodamine B protein and tetrazolium (MTT) assays for in vitro chemosensitivity testing. Eur J Cancer. 1991;27:897–900. - PubMed
-
- Hirai T, Kawano K, Hirabayashi N, Nishiyama M, Yamashita Y, Mukaida H, Iwata T, Toge T. A novel in vitro chemo-sensitivity test using materials collected by endoscopic biopsy. Anticancer Drugs. 1991;2:269–274. - PubMed
-
- Andreotti PE, Cree IA, Kurbacher CM, Hartmann DM, Linder D, Harel G, Gleiberman I, Caruso PA, Ricks SH, Untch M, et al. Chemosensitivity testing of human tumors using a microplate adenosine triphosphate luminescence assay: clinical correlation for cisplatin resistance of ovarian carcinoma. Cancer Res. 1995;55:5276–5282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

