Embryology and bony malformations of the craniovertebral junction
- PMID: 21193993
- PMCID: PMC3055990
- DOI: 10.1007/s00381-010-1358-9
Embryology and bony malformations of the craniovertebral junction
Abstract
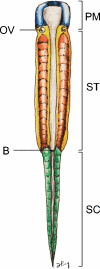
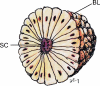
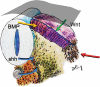
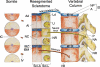
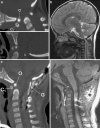
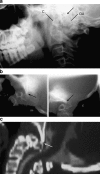
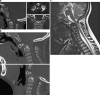
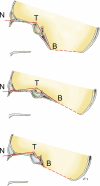
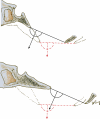
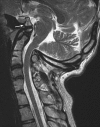
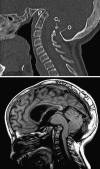
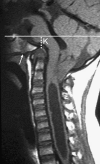
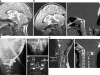
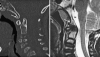
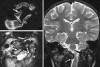
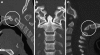
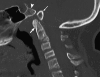
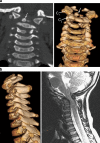
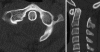
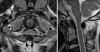
Background: The embryology of the bony craniovertebral junction (CVJ) is reviewed with the purpose of explaining the genesis and unusual configurations of the numerous congenital malformations in this region. Functionally, the bony CVJ can be divided into a central pillar consisting of the basiocciput and dental pivot and a two-tiered ring revolving round the central pivot, comprising the foramen magnum rim and occipital condyles above and the atlantal ring below. Embryologically, the central pillar and the surrounding rings descend from different primordia, and accordingly, developmental anomalies at the CVJ can also be segregated into those affecting the central pillar and those affecting the surrounding rings, respectively.
Discussion: A logical classification of this seemingly unwieldy group of malformations is thus possible based on their ontogenetic lineage, morbid anatomy, and clinical relevance. Representative examples of the main constituents of this classification scheme are given, and their surgical treatments are selectively discussed.
Figures






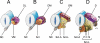



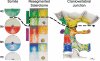





























References
-
- Aoyoma H, Asamoto K. Determination of somite cells: independence of cell differentiation and morphogenesis. Development. 1988;104:15–28. - PubMed
-
- Bellairs R. The segmentation of somites in the chick embryo. Bolletino di zoologia. 1980;47:245–252.
-
- Burwood RJ (1970) The cranio-cervical junction. Anatomy. Thesis. University of Bristol
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

