Serum biomarkers for improved diagnostic of pancreatic cancer: a current overview
- PMID: 21193998
- PMCID: PMC11827947
- DOI: 10.1007/s00432-010-0965-x
Serum biomarkers for improved diagnostic of pancreatic cancer: a current overview
Abstract
Purpose: Complete resection constitutes the only curative approach in pancreatic cancer but is possible only in a minority of patients due to advanced stages upon diagnosis. Consequently, early detection is crucial for curative treatment. Clinical routine still lacks efficient, non-invasive screening assays, and 80-90% of pancreatic carcinomas are detected at unresectable stages. A wide range of serum proteins have been in the focus of intensive search for biomarkers specific for pancreatic cancer. This article will give an overview on serum biomarkers with screening potential for pancreatic malignancy.
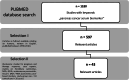
Design and methods: PUBMED database was searched for articles, and 43 manuscripts were selected that provided data regarding biomarkers used, type of assay, study population, sample cohort quality and diagnostic performance.
Results: Superior values for diagnostic performance were shown for MIC-1, PAM4, OPN, HSP27, TPS, TSGF, and CAM17.1 as individual markers. Panels of biomarkers comprised CA 19-9, MCSF, CEA, SAA, Haptoglobin, TSGF, CA 242, and HSP27. Individually or in concerted form, sensitivity and specificity ranged from 77 to 100% and 84-100%, respectively.
Conclusions: While the above named markers show high screening potential for pancreatic cancer, standardized validation studies using multiplex assays are required to pave the way for clinical routine application.
Conflict of interest statement
None.
Figures
References
-
- Banfi G, Zerbi A, Pastori S, Parolini D, Di Carlo V, Bonini P (1993) Behavior of tumor markers CA19.9, CA195, CAM43, CA242, and TPS in the diagnosis and follow-up of pancreatic cancer. Clin Chem 39:420–423 - PubMed
-
- Benson AB 3rd (2007) Adjuvant therapy for pancreatic cancer: one small step forward. JAMA 297:311–313 - PubMed
-
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC (2003) Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Standards for Reporting of Diagnostic Accuracy. Clin Chem 49:1–6 - PubMed
-
- Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, Aach J, Ansorge W, Ball CA, Causton HC et al (2001) Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nat Genet 29:365–371 - PubMed
-
- Brenner H, Hakulinen T (2009) Up-to-date cancer survival: period analysis and beyond. Int J Cancer 124:1384–1390 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous