GABA(A) receptors as molecular targets of general anesthetics: identification of binding sites provides clues to allosteric modulation
- PMID: 21194017
- PMCID: PMC3033524
- DOI: 10.1007/s12630-010-9429-7
GABA(A) receptors as molecular targets of general anesthetics: identification of binding sites provides clues to allosteric modulation
Abstract
Purpose: The purpose of this review is to summarize current knowledge of detailed biochemical evidence for the role of γ-aminobutyric acid type A receptors (GABA(A)-Rs) in the mechanisms of general anesthesia.
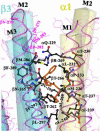
Principal findings: With the knowledge that all general anesthetics positively modulate GABA(A)-R-mediated inhibitory transmission, site-directed mutagenesis comparing sequences of GABA(A)-R subunits of varying sensitivity led to identification of amino acid residues in the transmembrane domain that are critical for the drug actions in vitro. Using a photo incorporable analogue of the general anesthetic, R(+)etomidate, we identified two transmembrane amino acids that were affinity labelled in purified bovine brain GABA(A)-R. Homology protein structural modelling positions these two residues, αM1-11' and βM3-4', close to each other in a single type of intersubunit etomidate binding pocket at the β/α interface. This position would be appropriate for modulation of agonist channel gating. Overall, available information suggests that these two etomidate binding residues are allosterically coupled to sites of action of steroids, barbiturates, volatile agents, and propofol, but not alcohols. Residue α/βM2-15' is probably not a binding site but allosterically coupled to action of volatile agents, alcohols, and intravenous agents, and α/βM1-(-2') is coupled to action of intravenous agents.
Conclusions: Establishment of a coherent and consistent structural model of the GABA(A)-R lends support to the conclusion that general anesthetics can modulate function by binding to appropriate domains on the protein. Genetic engineering of mice with mutation in some of these GABA(A)-R residues are insensitive to general anesthetics in vivo, suggesting that further analysis of these domains could lead to development of more potent and specific drugs.
Objectif: L’objectif de cet article de synthèse est de résumer les connaissances actuelles concernant les données probantes biochimiques détaillées élucidant le rôle des récepteurs à l’acide γ-aminobutyrique de type A (R-GABAA) dans les mécanismes de l’anesthésie générale.
Constatations principales: Tous les anesthésiques généraux modulent positivement la transmission inhibitrice médiée par les R-GABAA. On a identifié les acides aminés du domaine transmembranaire représentant des sites d’action importants des médicaments, en effectuant des mutations ciblées sur des séquences de sous-unités des R-GABAA et en comparant leur sensibilité. À l’aide d’un analogue photo luminescent de l’anesthésique général étomidate R(+), nous avons identifié deux acides aminés transmembranaires marquées par affinité dans les R-GABAA purifiés de cerveau bovin. Un modèle structurel de protéine par homologie place ces deux résidus, soit αM1-11’ et βM3-4’, à proximité l’un de l’autre dans un type unique de poche de liaison d’étomidate inter-sous-unité au niveau de l’interface β/α. Cette position sera adaptée pour moduler le portillon des canaux agonistes. Globalement, les données disponibles suggèrent que des deux résidus se liant à l’étomidate sont couplés de façon allostérique aux sites d’action des stéroïdes, des barbituriques, des agents volatils et du propofol, mais pas à ceux des alcools. Le résidu α/βM2-15’ n’est probablement pas un site de liaison, mais il est couplé de façon allostérique à l’action des agents volatils, des alcools et des agents intraveineux, et le α/βM1-(-2’) est couplé à l’action des agents intraveineux.
Conclusion: La création d’un modèle structurel cohérent et logique des R-GABAA appuie notre conclusion selon laquelle les anesthésiques généraux peuvent moduler leur fonction en se liant à des domaines spécifiques sur la protéine. Les souris génétiquement modifiées porteuses d’une mutation dans certains de ces résidus de R-GABAA ne sont pas sensibles aux anesthésiques généraux in vivo, ce qui suggère qu’une analyse plus approfondie de ces domaines pourrait permettre la mise au point de médicaments à la fois plus puissants et plus spécifiques.
Figures

Similar articles
-
Anesthetic sites and allosteric mechanisms of action on Cys-loop ligand-gated ion channels.Can J Anaesth. 2011 Feb;58(2):191-205. doi: 10.1007/s12630-010-9419-9. Epub 2011 Jan 7. Can J Anaesth. 2011. PMID: 21213095 Free PMC article. Review.
-
Mapping general anesthetic binding site(s) in human α1β3 γ-aminobutyric acid type A receptors with [³H]TDBzl-etomidate, a photoreactive etomidate analogue.Biochemistry. 2012 Jan 31;51(4):836-47. doi: 10.1021/bi201772m. Epub 2012 Jan 23. Biochemistry. 2012. PMID: 22243422 Free PMC article.
-
Numerous classes of general anesthetics inhibit etomidate binding to gamma-aminobutyric acid type A (GABAA) receptors.J Biol Chem. 2010 Mar 19;285(12):8615-20. doi: 10.1074/jbc.M109.074708. Epub 2010 Jan 18. J Biol Chem. 2010. PMID: 20083606 Free PMC article.
-
Neurosteroids allosterically modulate binding of the anesthetic etomidate to gamma-aminobutyric acid type A receptors.J Biol Chem. 2009 May 1;284(18):11771-5. doi: 10.1074/jbc.C900016200. Epub 2009 Mar 12. J Biol Chem. 2009. PMID: 19282280 Free PMC article.
-
Comparison of αβδ and αβγ GABAA receptors: Allosteric modulation and identification of subunit arrangement by site-selective general anesthetics.Pharmacol Res. 2018 Jul;133:289-300. doi: 10.1016/j.phrs.2017.12.031. Epub 2017 Dec 30. Pharmacol Res. 2018. PMID: 29294355 Free PMC article. Review.
Cited by
-
Structural models of ligand-gated ion channels: sites of action for anesthetics and ethanol.Alcohol Clin Exp Res. 2014 Mar;38(3):595-603. doi: 10.1111/acer.12283. Epub 2013 Oct 24. Alcohol Clin Exp Res. 2014. PMID: 24164436 Free PMC article. Review.
-
Human α1β3γ2L gamma-aminobutyric acid type A receptors: High-level production and purification in a functional state.Protein Sci. 2014 Feb;23(2):157-66. doi: 10.1002/pro.2401. Epub 2013 Dec 16. Protein Sci. 2014. PMID: 24288268 Free PMC article.
-
Effect of (+)-dehydrofukinone on GABAA receptors and stress response in fish model.Braz J Med Biol Res. 2016 Jan;49(1):e4872. doi: 10.1590/1414-431X20154872. Epub 2015 Nov 27. Braz J Med Biol Res. 2016. PMID: 26628396 Free PMC article.
-
Neurodevelopmental implications of the general anesthesia in neonate and infants.Exp Neurol. 2015 Oct;272:50-60. doi: 10.1016/j.expneurol.2015.03.028. Epub 2015 Apr 8. Exp Neurol. 2015. PMID: 25862287 Free PMC article. Review.
-
Functional role of translocator protein and its ligands in ocular diseases (Review).Mol Med Rep. 2024 Feb;29(2):33. doi: 10.3892/mmr.2024.13157. Epub 2024 Jan 8. Mol Med Rep. 2024. PMID: 38186312 Free PMC article. Review.
References
-
- Macdonald RL, Olsen RW. GABAA receptor channels. Ann Rev Neurosci. 1994;17:569–602. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

