Sulfated glycosaminoglycans accelerate transthyretin amyloidogenesis by quaternary structural conversion
- PMID: 21194234
- PMCID: PMC3035766
- DOI: 10.1021/bi101822y
Sulfated glycosaminoglycans accelerate transthyretin amyloidogenesis by quaternary structural conversion
Abstract
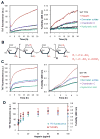
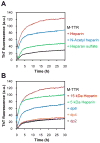
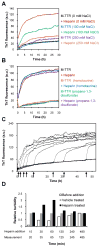
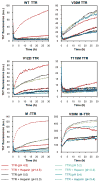
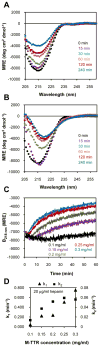
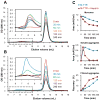
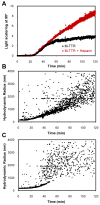
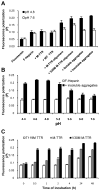
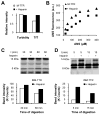
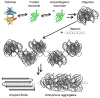
Glycosaminoglycans (GAGs), which are found in association with all extracellular amyloid deposits in humans, are known to accelerate the aggregation of various amyloidogenic proteins in vitro. However, the precise molecular mechanism(s) by which GAGs accelerate amyloidogenesis remains elusive. Herein, we show that sulfated GAGs, especially heparin, accelerate transthyretin (TTR) amyloidogenesis by quaternary structural conversion. The clustering of sulfate groups on heparin and its polymeric nature are essential features for accelerating TTR amyloidogenesis. Heparin does not influence TTR tetramer stability or TTR dissociation kinetics, nor does it alter the folded monomer-misfolded monomer equilibrium directly. Instead, heparin accelerates the conversion of preformed TTR oligomers into larger aggregates. The more rapid disappearance of monomeric TTR in the presence of heparin likely reflects the fact that the monomer-misfolded amyloidogenic monomer-oligomer-TTR fibril equilibria are all linked, a hypothesis that is strongly supported by the light scattering data. TTR aggregates prepared in the presence of heparin exhibit a higher resistance to trypsin and proteinase K proteolysis and a lower exposure of hydrophobic side chains comprising hydrophobic clusters, suggesting an active role for heparin in amyloidogenesis. Our data suggest that heparin accelerates TTR aggregation by a scaffold-based mechanism, in which the sulfate groups comprising GAGs interact primarily with TTR oligomers through electrostatic interactions, concentrating and orienting the oligomers, facilitating the formation of higher molecular weight aggregates. This model raises the possibility that GAGs may play a protective role in human amyloid diseases by interacting with proteotoxic oligomers and promoting their association into less toxic amyloid fibrils.
Figures
References
-
- Buxbaum JN. The systemic amyloidoses. Curr Opin Rheumatol. 2004;16:67–75. - PubMed
-
- Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. - PubMed
-
- Kelly JW. The alternative conformations of amyloidogenic proteins and their multistep assembly pathways. Curr Opin Struct Biol. 1998;8:101–106. - PubMed
-
- Dobson CM. Protein folding and misfolding. Nature. 2003;426:884–890. - PubMed
-
- Cohen FE, Kelly JW. Therapeutic approaches to protein-misfolding diseases. Nature. 2003;426:905–909. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

