Both maximal expression of selenoproteins and selenoprotein deficiency can promote development of type 2 diabetes-like phenotype in mice
- PMID: 21194350
- PMCID: PMC3096499
- DOI: 10.1089/ars.2010.3526
Both maximal expression of selenoproteins and selenoprotein deficiency can promote development of type 2 diabetes-like phenotype in mice
Abstract
Selenium (Se) is an essential trace element in mammals that has been shown to exert its function through selenoproteins. Whereas optimal levels of Se in the diet have important health benefits, a recent clinical trial has suggested that supplemental intake of Se above the adequate level potentially may raise the risk of type 2 diabetes mellitus. However, the molecular mechanisms for the effect of dietary Se on the development of this disease are not understood. In the present study, we examined the contribution of selenoproteins to increased risk of developing diabetes using animal models. C57BL/6J mice (n=6-7 per group) were fed either Se-deficient Torula yeast-based diet or diets supplemented with 0.1 and 0.4 parts per million Se. Our data show that mice maintained on an Se-supplemented diet develop hyperinsulinemia and have decreased insulin sensitivity. These effects are accompanied by elevated expression of a selective group of selenoproteins. We also observed that reduced synthesis of these selenoproteins caused by overexpression of an i(6)A(-) mutant selenocysteine tRNA promotes glucose intolerance and leads to a diabetes-like phenotype. These findings indicate that both high expression of selenoproteins and selenoprotein deficiency may dysregulate glucose homeostasis and suggest a role for selenoproteins in development of diabetes.
Figures
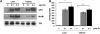
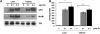






References
-
- Behne D. Hilmert H. Scheid S. Gessner H. Elger W. Evidence for specific selenium target tissues and new biologically important selenoproteins. Biochim Biophys Acta. 1988;966:12–21. - PubMed
-
- Bleys J. Navas-Acien A. Guallar E. Selenium and diabetes: more bad news for supplements. Ann Intern Med. 2007;147:271–272. - PubMed
-
- Blot WJ. Li JY. Taylor PR. Guo W. Dawsey S. Wang GQ. Yang CS. Zheng SF. Gail M. Li GY. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85:1483–1492. - PubMed
-
- Carlson BA. Xu XM. Shrimali R. Sengupta A. Yoo MH. Zhong N. Hatfield DL. Irons R. Davis CD. Lee BJ. Novoselov SV. Gladyshev VN. Mouse models for assessing the role of selenoproteins in health and development. In: Hatfield DL, editor; Berry MJ, editor; Gladyshev VN, editor. Selenium: Its Molecular Biology and Role in Human Health. New York, NY: Springer; 2006. pp. 333–341.
-
- Cheng WH. Ho YS. Ross DA. Han Y. Combs GF., Jr. Lei XG. Overexpression of cellular glutathione peroxidase does not affect expression of plasma glutathione peroxidase or phospholipid hydroperoxide glutathione peroxidase in mice offered diets adequate or deficient in selenium. J Nutr. 1997;127:675–680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

