Targeting mitochondrial dysfunction: role for PINK1 and Parkin in mitochondrial quality control
- PMID: 21194381
- PMCID: PMC3078490
- DOI: 10.1089/ars.2010.3799
Targeting mitochondrial dysfunction: role for PINK1 and Parkin in mitochondrial quality control
Abstract
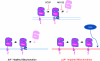
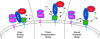
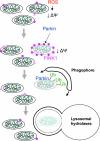
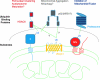
Mitochondria, which convert energy for the cell, accumulate damage with age, and the resulting mitochondrial dysfunction has been linked to the development of degenerative diseases and aging. To curb the accumulation of damaged mitochondria, the cell has elaborated a number of mitochondrial quality control processes. We describe recent work suggesting that Parkin and PTEN-induced putative kinase 1 (PINK1), two gene products linked to familial forms of parkinsonism, may constitute one of the cell's mitochondrial quality control pathways-identifying impaired mitochondria and selectively trimming them from the mitochondrial network by mitophagy. In particular, we discuss the regulation of PINK1 protein expression and Parkin localization by the bioenergetic status of individual mitochondria; the mechanism by which PINK1 recruits Parkin to the outer mitochondrial membrane; and Parkin's promotion of mitophagy through its ubiquitination of outer mitochondrial membrane proteins. This recent work suggests that Parkin and PINK1 may be among the first mammalian proteins identified with a direct role in regulating mitophagy, and implicate a failure of mitophagy in the pathogenesis of Parkinson's disease.
Figures

References
-
- Arnold I. Langer T. Membrane protein degradation by AAA proteases in mitochondria. Biochim Biophys Acta. 2002;1592:89–96. - PubMed
-
- Chen H. Chan DC. Physiological functions of mitochondrial fusion. Ann N Y Acad Sci. 2010;1201:21–25. - PubMed
-
- Chung KK. Zhang Y. Lim KL. Tanaka Y. Huang H. Gao J. Ross CA. Dawson VL. Dawson TM. Parkin ubiquitinates the alpha-synuclein-interacting protein, synphilin-1: implications for Lewy-body formation in Parkinson disease. Nat Med. 2001;7:1144–1150. - PubMed
-
- Clark IE. Dodson MW. Jiang C. Cao JH. Huh JR. Seol JH. Yoo SJ. Hay BA. Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

