A selective neurokinin-1 receptor antagonist in chronic PTSD: a randomized, double-blind, placebo-controlled, proof-of-concept trial
- PMID: 21194898
- PMCID: PMC3478767
- DOI: 10.1016/j.euroneuro.2010.11.012
A selective neurokinin-1 receptor antagonist in chronic PTSD: a randomized, double-blind, placebo-controlled, proof-of-concept trial
Abstract
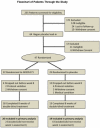
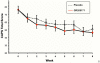
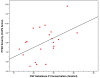
The substance P-neurokinin-1 receptor (SP-NK(1)R) system has been extensively studied in experimental models of stress, fear, and reward. Elevated cerebrospinal fluid (CSF) SP levels were reported previously in combat-related PTSD. No medication specifically targeting this system has been tested in PTSD. This proof-of-concept randomized, double-blind, placebo-controlled trial evaluated the selective NK(1)R antagonist GR205171 in predominately civilian PTSD. Following a 2-week placebo lead-in, 39 outpatients with chronic PTSD and a Clinician-Administered PTSD Scale (CAPS) score ≥50 were randomized to a fixed dose of GR205171 (N=20) or placebo (N=19) for 8weeks. The primary endpoint was mean change from baseline to endpoint in the total CAPS score. Response rate (≥50% reduction in baseline CAPS) and safety/tolerability were secondary endpoints. CSF SP concentrations were measured in a subgroup of patients prior to randomization. There was significant improvement in the mean CAPS total score across all patients over time, but no significant difference was found between GR205171 and placebo. Likewise, there was no significant effect of drug on the proportion of responders [40% GR205171 versus 21% placebo (p=0.30)]. An exploratory analysis showed that GR205171 treatment was associated with significant improvement compared to placebo on the CAPS hyperarousal symptom cluster. GR205171 was well-tolerated, with no discontinuations due to adverse events. CSF SP concentrations were positively correlated with baseline CAPS severity. The selective NK(1)R antagonist GR205171 had fewer adverse effects but was not significantly superior to placebo in the short-term treatment of chronic PTSD. (ClinicalTrials.gov Identifier: NCT 00211861, NCT 00383786).
Trial registration: ClinicalTrials.gov NCT00211861 NCT00383786.
Published by Elsevier B.V.
Figures
References
-
- Benedek DM, Friedman MJ, Zatzick D, Ursano RJ. American Psychiatric Association Practice Guidelines for the Treatment of Psychiatric Disorders. 2004. Practice Guideline for the Treatment of Patients with Acute Stress Disorder and Postraumatic Stress Disorder. - PubMed
-
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. - PubMed
-
- Committee on Treatment of Posttraumatic Stress Disorder (Institute of Medicine) Treatment of Posttraumatic Stress Disorder: An Assessment of the Evidence. National Academies Press; Washington, DC: 2007.
-
- Davidson JRT. Remission in post-traumatic stress disorder (PTSD): effects of sertraline as assessed by the Davidson Trauma Scale, Clinical Global Impressions and the Clinician-Administered PTSD scale. Int Clin Psychopharmacology. 2004;19:85–87. - PubMed
-
- Davidson JR, Book SW, Colket JT, Tupler LA, et al. Assessment of a new self-rating scale for post-traumatic stress disorder. Psychol Med. 1997;27:153–160. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

