CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut
- PMID: 21194981
- PMCID: PMC3035987
- DOI: 10.1016/j.immuni.2010.12.009
CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut
Abstract
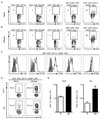
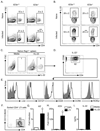
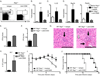
Fetal CD4(+) lymphoid tissue inducer (LTi) cells play a critical role in the development of lymphoid tissues. Recent studies identified that LTi cells persist in adults and are related to a heterogeneous population of innate lymphoid cells that have been implicated in inflammatory responses. However, whether LTi cells contribute to protective immunity remains poorly defined. We demonstrate that after infection with Citrobacter rodentium, CD4(+) LTi cells were a dominant source of interleukin-22 (IL-22) early during infection. Infection-induced CD4(+) LTi cell responses were IL-23 dependent, and ablation of IL-23 impaired innate immunity. Further, depletion of CD4(+) LTi cells abrogated infection-induced expression of IL-22 and antimicrobial peptides, resulting in exacerbated host mortality. LTi cells were also found to be essential for host protective immunity in lymphocyte-replete hosts. Collectively these data demonstrate that adult CD4(+) LTi cells are a critical source of IL-22 and identify a previously unrecognized function for CD4(+) LTi cells in promoting innate immunity in the intestine.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no further conflicting financial interests.
Figures




References
-
- Bonnet F, Chene G, Thiebaut R, Dupon M, Lawson-Ayayi S, Pellegrin JL, Dabis F, Morlat P. Trends and determinants of severe morbidity in HIV-infected patients: the ANRS CO3 Aquitaine Cohort, 2000–2004. HIV Med. 2007;8:547–554. - PubMed
-
- Burnet FM. Evolution of the immune process in vertebrates. Nature. 1968;218:426–430. - PubMed
-
- Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31:15–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI074878/AI/NIAID NIH HHS/United States
- T32AI007532-08/AI/NIAID NIH HHS/United States
- R01 AI095466/AI/NIAID NIH HHS/United States
- DK50306/DK/NIDDK NIH HHS/United States
- AI087990/AI/NIAID NIH HHS/United States
- AI083480/AI/NIAID NIH HHS/United States
- AI74878/AI/NIAID NIH HHS/United States
- R21 AI087990/AI/NIAID NIH HHS/United States
- R21 AI083480/AI/NIAID NIH HHS/United States
- R01 AI061570/AI/NIAID NIH HHS/United States
- AI61570/AI/NIAID NIH HHS/United States
- P30 DK050306/DK/NIDDK NIH HHS/United States
- T32 AI007532/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

