Ferric ions inhibit proteolytic processing of progastrin
- PMID: 21195058
- PMCID: PMC3046803
- DOI: 10.1016/j.bbrc.2010.12.117
Ferric ions inhibit proteolytic processing of progastrin
Abstract
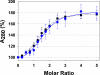
The gastrointestinal hormone gastrin is generated from an 80 amino acid precursor (progastrin) by cleavage after dibasic residues by prohormone convertase 1. Phosphorylation of Ser(75) has previously been suggested, on the basis of indirect evidence, to inhibit cleavage of progastrin after Arg(73)Arg(74). Gastrins bind two ferric ions with high affinity, and iron binding is essential for the biological activity of non-amidated gastrins in vitro and in vivo. This study directly investigated the effect of iron binding and of serine phosphorylation on the cleavage of synthetic progastrin-derived peptides. The affinity of synthetic progastrin(55-80) for ferric ions, and the rate of cleavage by prohormone convertase 1, were not affected by phosphorylation of Ser(75). In contrast, in the presence of ferric ions the rate of cleavage of both progastrin(55-80) and phosphoSer(75)progastrin(55-80) by prohormone convertase 1 was significantly reduced. Hence iron binding to progastrin may regulate processing and secretion in vivo, and regulation may be particularly important in diseases with altered iron homeostasis.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
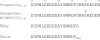


Similar articles
-
Prohormone convertases 1/3 and 2 together orchestrate the site-specific cleavages of progastrin to release gastrin-34 and gastrin-17.Biochem J. 2008 Oct 1;415(1):35-43. doi: 10.1042/BJ20080881. Biochem J. 2008. PMID: 18554181
-
Diminished prohormone convertase 3 expression (PC1/PC3) inhibits progastrin post-translational processing.Regul Pept. 2000 May 10;89(1-3):19-28. doi: 10.1016/s0167-0115(99)00126-3. Regul Pept. 2000. PMID: 10771309
-
Regulation by gastric acid of the processing of progastrin-derived peptides in rat antral mucosa.J Physiol. 1997 Jul 15;502 ( Pt 2)(Pt 2):409-19. doi: 10.1111/j.1469-7793.1997.409bk.x. J Physiol. 1997. PMID: 9263920 Free PMC article.
-
Naming progastrin-derived peptides.Regul Pept. 2004 Aug 15;120(1-3):177-83. doi: 10.1016/j.regpep.2004.03.006. Regul Pept. 2004. PMID: 15177936 Review.
-
Posttranslational processing of progastrin.Results Probl Cell Differ. 2010;50:207-20. doi: 10.1007/400_2009_34. Results Probl Cell Differ. 2010. PMID: 19960379 Review.
Cited by
-
Bismuth ions inhibit the biological activity of non-amidated gastrins in vivo.Biochem Pharmacol. 2012 Feb 15;83(4):524-30. doi: 10.1016/j.bcp.2011.11.030. Epub 2011 Dec 8. Biochem Pharmacol. 2012. PMID: 22172990 Free PMC article.
-
Novel roles of gastrin.J Physiol. 2014 Jul 15;592(14):2951-8. doi: 10.1113/jphysiol.2014.272435. Epub 2014 Mar 24. J Physiol. 2014. PMID: 24665102 Free PMC article. Review.
References
-
- Varro A, Nemeth J, Bridson J, Lee C, Moore S, Dockray GJ. Processing of the gastrin precursor. Modulation of phosphorylated, sulfated, and amidated products. J. Biol. Chem. 1990;265:21476–21481. - PubMed
-
- Walsh JH. Peptides as regulators of gastric acid secretion. Annu. Rev. Physiol. 1988;50:41–63. - PubMed
-
- Hollande F, Imdahl A, Mantamadiotis T, Ciccotosto GD, Shulkes A, Baldwin GS. Glycine-extended gastrin acts as an autocrine growth factor in a nontransformed colon cell line. Gastroenterology. 1997;113:1576–1588. - PubMed
-
- Dockray GJ, Varro A, Desmond H, Young J, Gregory H, Gregory RA. Post-translational processing of the porcine gastrin precursor by phosphorylation of the COOH-terminal fragment. J. Biol. Chem. 1987;262:8643–8647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

