Comparison of immune responses generated by optimized DNA vaccination against SIV antigens in mice and macaques
- PMID: 21195080
- PMCID: PMC3115438
- DOI: 10.1016/j.vaccine.2010.12.056
Comparison of immune responses generated by optimized DNA vaccination against SIV antigens in mice and macaques
Abstract
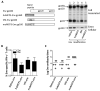
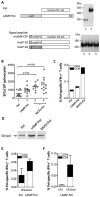
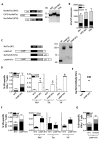
Optimized DNA vectors were constructed comprising the proteome of SIV including the structural, enzymatic, regulatory, and accessory proteins. In addition to native antigens as produced by the virus, fusion proteins and modified antigens with altered secretion, cellular localization and stability characteristics were generated. The DNA vectors were tested for expression upon transfection in human cells. In addition, the vectors were tested either alone or in combinations in mice and macaques, which provided an opportunity to compare immune responses in two animal models. DNA only immunization using intramuscular injection in the absence or presence of in vivo electroporation did not alter the phenotype of the induced T cell responses in mice. Although several fusion proteins induced immune responses to all the components of a polyprotein, we noted fusion proteins that abrogated immune response to some of the components. Since the expression levels of such fusion proteins were not affected, these data suggest that the immune recognition of certain components was altered by the fusion. Testing different DNA vectors in mice and macaques revealed that a combination of DNAs producing different forms of the same antigen generated more balanced immune responses, a desirable feature for an optimal AIDS vaccine.
Published by Elsevier Ltd.
Figures



Similar articles
-
Dose-dependent inhibition of Gag cellular immunity by Env in SIV/HIV DNA vaccinated macaques.Hum Vaccin Immunother. 2015;11(8):2005-11. doi: 10.1080/21645515.2015.1016671. Hum Vaccin Immunother. 2015. PMID: 26125521 Free PMC article.
-
Comparative efficacy of recombinant modified vaccinia virus Ankara expressing simian immunodeficiency virus (SIV) Gag-Pol and/or Env in macaques challenged with pathogenic SIV.J Virol. 2000 Mar;74(6):2740-51. doi: 10.1128/jvi.74.6.2740-2751.2000. J Virol. 2000. PMID: 10684290 Free PMC article.
-
Vaccination with Vaxfectin(®) adjuvanted SIV DNA induces long-lasting humoral immune responses able to reduce SIVmac251 Viremia.Hum Vaccin Immunother. 2013 Oct;9(10):2069-80. doi: 10.4161/hv.25442. Epub 2013 Jul 2. Hum Vaccin Immunother. 2013. PMID: 23820294 Free PMC article.
-
Recombinant rubella vectors elicit SIV Gag-specific T cell responses with cytotoxic potential in rhesus macaques.Vaccine. 2015 Apr 27;33(18):2167-74. doi: 10.1016/j.vaccine.2015.02.067. Epub 2015 Mar 21. Vaccine. 2015. PMID: 25802183 Free PMC article.
-
Virus-like particle and DNA-based candidate AIDS vaccines.Vaccine. 2003 Jan 30;21(7-8):638-43. doi: 10.1016/s0264-410x(02)00572-8. Vaccine. 2003. PMID: 12531332 Review.
Cited by
-
Comparative analysis of SIV-specific cellular immune responses induced by different vaccine platforms in rhesus macaques.Clin Immunol. 2014 Nov;155(1):91-107. doi: 10.1016/j.clim.2014.09.005. Epub 2014 Sep 16. Clin Immunol. 2014. PMID: 25229164 Free PMC article.
-
In vivo molecular imaging and histological analysis of changes induced by electric pulses used for plasmid DNA electrotransfer to the skin: a study in a dorsal window chamber in mice.J Membr Biol. 2012 Sep;245(9):545-54. doi: 10.1007/s00232-012-9435-5. Epub 2012 May 27. J Membr Biol. 2012. PMID: 22644389 Free PMC article.
-
A human immune data-informed vaccine concept elicits strong and broad T-cell specificities associated with HIV-1 control in mice and macaques.J Transl Med. 2015 Feb 15;13:60. doi: 10.1186/s12967-015-0392-5. J Transl Med. 2015. PMID: 25879820 Free PMC article.
-
Optimization of HIV-1 Envelope DNA Vaccine Candidates within Three Different Animal Models, Guinea Pigs, Rabbits and Cynomolgus Macaques.Vaccines (Basel). 2013 Jul 19;1(3):305-27. doi: 10.3390/vaccines1030305. Vaccines (Basel). 2013. PMID: 26344115 Free PMC article.
-
Live attenuated Rev-independent Nef¯SIV enhances acquisition of heterologous SIVsmE660 in acutely vaccinated rhesus macaques.PLoS One. 2013 Sep 30;8(9):e75556. doi: 10.1371/journal.pone.0075556. eCollection 2013. PLoS One. 2013. PMID: 24098702 Free PMC article.
References
-
- Catanzaro AT, Roederer M, Koup RA, Bailer RT, Enama ME, Nason MC, et al. Phase I clinical evaluation of a six-plasmid multiclade HIV-1 DNA candidate vaccine. Vaccine. 2007 May 16;25:4085–92. - PubMed
-
- MacGregor RR, Boyer JD, Ugen KE, Lacy KE, Gluckman SJ, Bagarazzi ML, et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J Infect Dis. 1998;178:92–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

