MCM3AP is transcribed from a promoter within an intron of the overlapping gene for GANP
- PMID: 21195085
- PMCID: PMC3121959
- DOI: 10.1016/j.jmb.2010.12.035
MCM3AP is transcribed from a promoter within an intron of the overlapping gene for GANP
Abstract
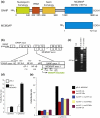
MCM3 acetylase (MCM3AP) and germinal-centre associated nuclear protein (GANP) are transcribed from the same locus and are therefore confused in databases because the MCM3 acetylase DNA sequence is contained entirely within the much larger GANP sequence and the entire MCM3AP sequence is identical to the carboxy terminus of GANP. Thus, the MCM3AP and GANP genes are read in the same reading frame and MCM3AP is an N-terminally truncated region of GANP. However, we show here that MCM3AP and GANP are different proteins, occupying different locations in the cell and transcribed from different promoters. Intriguingly, a promoter for MCM3AP lies within an intron of GANP. This report is an interesting example in nature of two separate gene products from the same locus that perform two entirely different functions in the cell. Therefore, to avoid further confusion, they should now be referred to as separate but overlapping genes.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Distinct effects on mRNA export factor GANP underlie neurological disease phenotypes and alter gene expression depending on intron content.Hum Mol Genet. 2020 Jun 3;29(9):1426-1439. doi: 10.1093/hmg/ddaa051. Hum Mol Genet. 2020. PMID: 32202298 Free PMC article.
-
Functional interaction between the glucocorticoid receptor and GANP/MCM3AP.Biochem Biophys Res Commun. 2006 Oct 6;348(4):1239-44. doi: 10.1016/j.bbrc.2006.07.182. Epub 2006 Aug 7. Biochem Biophys Res Commun. 2006. PMID: 16914116
-
The MCM3 acetylase MCM3AP inhibits initiation, but not elongation, of DNA replication via interaction with MCM3.J Biol Chem. 2002 Nov 8;277(45):43121-5. doi: 10.1074/jbc.C200442200. Epub 2002 Sep 10. J Biol Chem. 2002. PMID: 12226073
-
Germinal Center B-Cell-Associated Nuclear Protein (GANP) Involved in RNA Metabolism for B Cell Maturation.Adv Immunol. 2016;131:135-86. doi: 10.1016/bs.ai.2016.02.003. Epub 2016 Mar 29. Adv Immunol. 2016. PMID: 27235683 Review.
-
The critical role of germinal center-associated nuclear protein in cell biology, immunohematology, and hematolymphoid oncogenesis.Exp Hematol. 2020 Oct;90:30-38. doi: 10.1016/j.exphem.2020.08.007. Epub 2020 Aug 20. Exp Hematol. 2020. PMID: 32827560 Review.
Cited by
-
Identification of a novel caspase cleavage motif AEAD.Virol Sin. 2024 Oct;39(5):755-766. doi: 10.1016/j.virs.2024.08.001. Epub 2024 Aug 3. Virol Sin. 2024. PMID: 39098717 Free PMC article.
-
Tumorigenesis Caused by Aberrant Expression of GANP, a Central Component in the Mammalian TREX-2 Complex-Lessons from Transcription-Coupled DNA Damages.Int J Mol Sci. 2024 Dec 19;25(24):13612. doi: 10.3390/ijms252413612. Int J Mol Sci. 2024. PMID: 39769375 Free PMC article. Review.
-
The cellular protein MCM3AP is required for inhibition of cellular DNA synthesis by the IE86 protein of human cytomegalovirus.PLoS One. 2012;7(10):e45686. doi: 10.1371/journal.pone.0045686. Epub 2012 Oct 19. PLoS One. 2012. PMID: 23094019 Free PMC article.
-
The Human TREX-2 Complex Interacts with Subunits of the ORC Complex.Dokl Biochem Biophys. 2023 Dec;513(1):346-349. doi: 10.1134/S1607672923700552. Epub 2023 Dec 8. Dokl Biochem Biophys. 2023. PMID: 38066323 Free PMC article.
-
The Xmas-2 Protein of Drosophila melanogaster Undergoes Cleavage into Two Fragments.Dokl Biochem Biophys. 2023 Apr;509(1):37-40. doi: 10.1134/S1607672923700163. Epub 2023 Jun 20. Dokl Biochem Biophys. 2023. PMID: 37340289
References
-
- Takei Y., Tsujimoto G. Identification of a novel MCM3-associated protein that facilitates MCM3 nuclear localisation. J. Biol. Chem. 1998;273:22177–22180. - PubMed
-
- Takei Y., Assenberg M., Tsujimoto G., Laskey R. The MCM3 acetylase MCM3AP inhibits initiation, but not elongation, of DNA replication via interaction with MCM3. J. Biol. Chem. 2002;277:43121–43125. - PubMed
-
- Abe E., Kuwahara K., Yoshida M., Suzuki M., Terasaki H., Matsuo Y. Structure, expression and chromosomal localisation of the human gene encoding a germinal centre-associated nuclear protein (GANP) that associates with MCM3 involved in the initiation of DNA replication. Gene. 2000;255:219–227. - PubMed
-
- Fujimura S., Xing Y., Takeya M., Yamashita Y., Ohshima K., Kuwahara K., Sakaguchi N. Increased expression of germinal center-associated nuclear protein RNA-primase is associated with lymphomagenesis. Cancer Res. 2005;65:5925–5934. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

