Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis
- PMID: 21195352
- PMCID: PMC3038267
- DOI: 10.1016/j.cmet.2010.12.010
Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis
Erratum in
- Cell Metab. 2012 Jan 4;15(1):128
Abstract
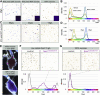
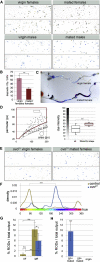
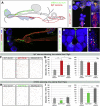
The gastrointestinal tract is emerging as a key regulator of appetite and metabolism, but daunting neuroanatomical complexity has hampered identification of the relevant signals. Invertebrate models could provide a simple and genetically amenable alternative, but their autonomic nervous system and its visceral functions remain largely unexplored. Here we develop a quantitative method based on defecation behavior to uncover a central role for the Drosophila intestine in the regulation of nutrient intake, fluid, and ion balance. We then identify a key homeostatic role for autonomic neurons and hormones, including a brain-gut circuit of insulin-producing neurons modulating appetite, a vasopressin-like system essential for fluid homeostasis, and enteric neurons mediating sex peptide-induced changes in intestinal physiology. These conserved mechanisms of visceral control, analogous to those found in the enteric nervous system and hypothalamic/pituitary axis, enable the study of autonomic control in a model organism that has proved instrumental in understanding sensory and motor systems.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures





References
-
- Agoston M.K. Springer; London: 2005. Computer Graphics and Geometric Modeling: Implementation and Algorithms.
-
- Arquier N., Geminard C., Bourouis M., Jarretou G., Honegger B., Paix A., Leopold P. Drosophila ALS regulates growth and metabolism through functional interaction with insulin-like peptides. Cell Metab. 2008;7:333–338. - PubMed
-
- Avery L., Thomas J.H. Feeding and defecation. In: Riddle D.L., Blumenthal T., Meyer B.J., Priess J.R., editors. C. elegans II. Cold Spring Harbor Laboratory Press; New York: 1997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

