Trans-iliac rat aorta stenting: a novel high throughput preclinical stent model for restenosis and thrombosis
- PMID: 21195423
- PMCID: PMC3071592
- DOI: 10.1016/j.jss.2010.11.882
Trans-iliac rat aorta stenting: a novel high throughput preclinical stent model for restenosis and thrombosis
Erratum in
- J Surg Res. 2012 May 1;174(1):184
Abstract
Background: Currently, preclinical stent development requires elaborate large animal models, which are time consuming and expensive. We herein report a high throughput rat aorta stenting model which could provide a rapid and low-cost platform for preclinical stent development.
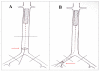
Methods: A total of 86 metal stents (316L stainless steel 13 mm, VasoTech, Inc.) coated with poly (D, L-lactide-co-glycolide)/amorphous calcium phosphate (PLGA/ACP) copolymer were pre-mounted on 1.5 mm × 15 mm balloon catheters and were implanted into aspirin treated Sprague-Dawley rats (500-700 g) initially using either direct placement in the abdominal aorta (group A, n = 7) or a trans-iliac approach (cut-down, group B, n = 79). The surviving rats were sacrificed at 1, 2, 4, and 12 wk post-implantation and the stented arteries were analyzed histopathologically.
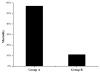
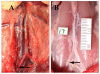
Results: Four rats died in group A and nine rats died in group B within 48 h post-stent implantation (mortality: 57% versus 11%, P < 0.05). All animals that died had stent thrombosis/paralysis with visible thrombus on necropsy. Histologically, neointimal growth peaked at approximately 4 wk post-implantation.
Conclusion: This result suggests that human-sized stents can be successfully implanted into the rat aorta via iliac artery insertion with a significantly higher survival rate than trans-aorta implantation. The model system allows rapid (4-12 wk) assessment of stent biocompatibility with mortality/paralysis used as an indicator of stent thrombosis.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


References
-
- van der Hoeven BL, Pires NMM, Warda HM, Oemrawsingh PV, van Vlijmen BJM, et al. Drug-eluting stents: results, promises and problems. Int J Cardiol. 2005;99:9–17. - PubMed
-
- Iijima R, Ikari Y, Miyazawa A, Nakajima H, Hara K. Predictors of restenosis after implantation of 2.5 mm stents in small coronary arteries. Circ J. 2004;68:236–240. - PubMed
-
- Schofer J, Schlüter M, Gershlick AH, Wijns W, García E, et al. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double-blind, randomised controlled trial (E-SIRIUS) Lancet. 2003;362:1093–1099. - PubMed
-
- Muramatsu T, Tsukahara R, Ho M, Ito Y, Ishimori H, et al. Clinical outcome of stent implantation in small coronary arteries using different type of coronary stents. J Invasive Cardiol. 2001;13:634–639. - PubMed
-
- Johnson GJ, Griggs TR, Badimon L. The utility of animal models in the preclinical study of interventions to prevent human coronary artery restenosis: on behalf of the subcommittee on animal, cellular and molecular models of thrombosis and haemostasis of the scientific and standardization committee of the international society of thrombosis and haemostasis. Thromb Haemost. 1999;81:835–843. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

