Toll-like receptor activation modulates antimicrobial peptide expression by ocular surface cells
- PMID: 21195713
- PMCID: PMC3049847
- DOI: 10.1016/j.exer.2010.12.005
Toll-like receptor activation modulates antimicrobial peptide expression by ocular surface cells
Abstract
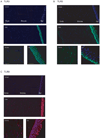
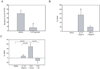
The ability of the ocular surface to respond to pathogens is in part attributed to toll-like receptors (TLRs) that recognize conserved motifs on various microbes. This study examines TLR expression on various ocular surface cells, if TLR agonists can modulate the expression of antimicrobial peptides (AMPs), human beta defensins (hBD) and cathelicidin (hCAP-18/LL-37) which maybe functionally active against Pseudomonas aeruginosa (PA) and if TLR agonists or AMPs can modulate TLR mRNA expression. TLR1-10 mRNA expression was examined in corneal epithelial, corneal stromal cells and conjunctival epithelial cells by RT-PCR. To confirm protein expression flow cytometry or immunostaining was performed for selected TLRs on some cell cultures. Ocular surface cells were cultured with a range of TLR agonists and then hBD-1, 2, 3, or hCAP-18 mRNA and protein expression was determined by RT-PCR and immunoblotting. In some experiments, cells were cultured with a cocktail of agonists for TLR3, 5 and 6/2 and the antimicrobial activity of the culture media was tested against PA. TLR mRNA expression was also examined in primary human corneal epithelial cells (HCEC) treated with either 3 μg/ml of hBD-2, 5 μg/ml of LL-37 or TLR4, 5 and 9 agonists. Overall, the ocular surface cells expressed mRNA for most of the TLRs but some differences were found. TLR2 was not detected in corneal fibroblasts, TLR4 was not detected in primary cultured or freshly isolated HCEC, TLR5 was not detected in conjunctival epithelial cells (IOBA-NHC) and corneal fibroblasts, TLR7 was not detected in freshly isolated HCEC and TLR10 was not detected in HCEC and IOBA-NHC. TLR8 mRNA was not expressed by any of the samples tested. Immunostaining of cadaver corneas revealed TLR5 and 9 expression throughout the cornea while TLR3 was significantly expressed only in the epithelium. Flow cytometry and immunostaining revealed cultured fibroblasts expressed TLR9 but had no significant TLR3 expression. hBD-2 expression was upregulated by TLR1/2, 3, 4, 5 and 6/2 agonists depending on the cell type, whereas only the TLR3 agonist upregulated the expression of hCAP-18 in primary HCEC. The combination of TLR3, 5 and 6/2 agonists in primary HCEC, upregulated hBD-2 and hCAP-18 mRNA and peptide expression and secretion into the culture media, which significantly killed PA. This antimicrobial activity was primarily attributed to LL-37. TLR agonists did not modulate TLR expression itself, however, LL-37 or hBD-2 downregulated TLR5, 7 and/or 9 mRNA depending on the cell type. TLRs are expressed on the ocular surface and TLR agonists trigger the production of LL-37 and hBD-2, with LL-37 being particularly important for protecting the ocular surface against PA infection.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures



References
-
- Aarbiou J, Ertmann M, van Wetering S, et al. Human neutrophil defensins induce lung epithelial cell proliferation in vitro. J.Leukoc.Biol. 2002;72:167–174. - PubMed
-
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. - PubMed
-
- Befus AD, Mowat C, Gilchrist M, Hu J, Solomon S, Bateman A. Neutrophil defensins induce histamine secretion from mast cells: mechanisms of action. J. Immunol. 1999;163:947–953. - PubMed
-
- Beutler B. TLR4 as the mammalian endotoxin sensor. Curr Top Microbiol Immunol. 2002;270:109–120. - PubMed
-
- Blais DR, Vascotto SG, Griffith M, Altosaar I. LBP and CD14 secreted in tears by the lacrimal glands modulate the LPS response of corneal epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:4235–4244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

