EGFR-homing dsRNA activates cancer-targeted immune response and eliminates disseminated EGFR-overexpressing tumors in mice
- PMID: 21196415
- PMCID: PMC3079342
- DOI: 10.1158/1078-0432.CCR-10-1140
EGFR-homing dsRNA activates cancer-targeted immune response and eliminates disseminated EGFR-overexpressing tumors in mice
Abstract
Purpose: The cause of most cancer deaths is incurable dissemination of cancer cells into vital organs. Current systemic therapies for disseminated cancers provide limited efficacy and are often accompanied by toxic side effects. We have recently shown that local application of epidermal growth factor receptor (EGFR)-targeted polyinosine-cytosine (polyIC) eradicates preestablished EGFR-overexpressing tumors. Here we show for the first time the high efficiency of systemic application of polyIC/melittin-polyethyleneimine-polyethyleneglycol-EGF (polyIC/MPPE) in combination with human immune cells.
Experimental design: Cancer-targeted activation of immune cells was examined in vitro and in vivo following transfection with polyIC/MPPE. The therapeutic efficiency of the strategy was then examined on disseminated EGFR-overexpressing tumors grown in severe combined immunodeficient (SCID) mice.
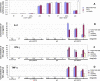
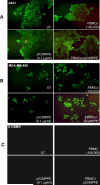
Results: Intravenous delivery of polyIC/MPPE followed by intraperitoneal injection of peripheral blood mononuclear cells induced the complete cure of SCID mice with preestablished disseminated EGFR-overexpressing tumors, with no adverse toxic effects. The immune cells and the cytokines they produce are localized to the tumor site of the treated animal and contribute decisively to the demise of the tumor cells. The immune system homes to the tumors, due to the chemokines produced by the internalized polyIC.
Conclusion: The EGFR-homing vector loaded with polyIC can be used to treat and possibly cure patients with disseminated EGFR-overexpressing tumors. The possibility of adopting this strategy to treat other tumors that express a protein capable of ligand induced internalization is discussed.
©2010 AACR.
Figures



References
-
- Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol. 2009;21:177–84. - PubMed
-
- Han Y, Caday CG, Nanda A, Cavenee WK, Huang HJ. Tyrphostin AG 1478 preferentially inhibits human glioma cells expressing truncated rather than wild-type epidermal growth factor receptors. Cancer Res. 1996;56:3859–61. - PubMed
-
- Kershaw MH, Wang G, Westwood JA, et al. Redirecting migration of T cells to chemokine secreted from tumors by genetic modification with CXCR2. Hum Gene Ther. 2002;13:1971–80. - PubMed
-
- Huang H, Xiang J. Synergistic effect of lymphotactin and interferon gamma-inducible protein-10 transgene expression in T-cell localization and adoptive T-cell therapy of tumors. Int J Cancer. 2004;109:817–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

