Immunodetection of retinoblastoma-related protein and its phosphorylated form in interphase and mitotic alfalfa cells
- PMID: 21196474
- PMCID: PMC3060694
- DOI: 10.1093/jxb/erq413
Immunodetection of retinoblastoma-related protein and its phosphorylated form in interphase and mitotic alfalfa cells
Abstract
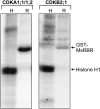
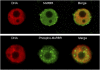
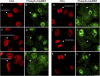
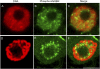
Plant retinoblastoma-related (RBR) proteins are primarily considered as key regulators of G(1)/S phase transition, with functional roles in a variety of cellular events during plant growth and organ development. Polyclonal antibody against the C-terminal region of the Arabidopsis RBR1 protein also specifically recognizes the alfalfa 115 kDa MsRBR protein, as shown by the antigen competition assay. The MsRBR protein was detected in all cell cycle phases, with a moderate increase in samples representing G(2)/M cells. Antibody against the human phospho-pRb peptide (Ser807/811) cross-reacted with the same 115 kDa MsRBR protein and with the in vitro phosphorylated MsRBR protein C-terminal fragment. Phospho-MsRBR protein was low in G(1) cells. Its amount increased upon entry into the S phase and remained high during the G(2)/M phases. Roscovitine treatment abolished the activity of alfalfa MsCDKA1;1 and MsCDKB2;1, and the phospho-MsRBR protein level was significantly decreased in the treated cells. Colchicine block increased the detected levels of both forms of MsRBR protein. Reduced levels of the MsRBR protein in cells at stationary phase or grown in hormone-free medium can be a sign of the division-dependent presence of plant RBR proteins. Immunolocalization of the phospho-MsRBR protein indicated spots of variable number and size in the labelled interphase nuclei and high signal intensity of nuclear granules in prophase. Structures similar to phospho-MsRBR proteins cannot be recognized in later mitotic phases. Based on the presented western blot and immunolocalization data, the possible involvement of RBR proteins in G(2)/M phase regulation in plant cells is discussed.
Figures





References
-
- Berckmans B, De Veylder L. Transcriptional control of the cell cycle. Current Opinion in Plant Biology. 2009;12:599–605. - PubMed
-
- Bhattacharyya B, Panda D, Gupta S, Banerjee M. Anti-mitotic activity of colchicine and the structural basis for its interaction with tubulin. Medicinal Research Reviews. 2008;28:155–183. - PubMed
-
- Blom N, Sicheritz-Ponten T, Gupta R, Gammeltoft S, Brunak S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics. 2004;4:1633–1649. - PubMed
-
- Bögre L, Oláh Z, Dudits D. Ca2-dependent protein kinase from alfalfa (Medicago varia): partial purification and autophosphorylation. Plant Science. 1988;58:135–144.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

