Comparison of phenotypic and genotypic tropism determination in triple-class-experienced HIV patients eligible for maraviroc treatment
- PMID: 21196489
- PMCID: PMC3019088
- DOI: 10.1093/jac/dkq458
Comparison of phenotypic and genotypic tropism determination in triple-class-experienced HIV patients eligible for maraviroc treatment
Abstract
Background: Determination of HIV-1 tropism is a pre-requisite to the use of CCR5 antagonists. This study evaluated the potential of population genotypic tropism tests (GTTs) in clinical practice, and the correlation with phenotypic tropism tests (PTTs) in patients accessing routine HIV care.
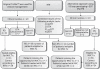
Methods: Forty-nine consecutive plasma samples for which an original Trofile(TM) assay was performed were obtained from triple-class-experienced patients in need of a therapy change. Viral tropism was defined as the consensus of three or more tropism calls obtained from the combination of two independent population PTT assays (Trofile Biosciences, San Francisco, CA, USA, and Virco, Beerse, Belgium), population GTTs and GTTs based on ultra-deep sequencing. If no consensus was reached, a clonal PTT was performed in order to finalize the tropism call. This two-step approach allowed the definition of a reference tropism call.
Results: According to the reference tropism result, 35/49 samples were CCR5 tropic (R5) (patients eligible for maraviroc treatment) and 14/49 were assigned as non-R5 tropic. The non-R5 samples [patients not eligible for maraviroc treatment according to the FDA/European Medicines Agency (EMEA) label] group included both the CXCR4 (X4) samples and the dual and mixed CCR5/CXCR4 (R5/X4) samples. Compared with Trofile(TM) population PTTs, population GTTs showed a higher sensitivity (97%) and a higher negative predictive value (91%), but almost equal specificity and an equal positive predictive value.
Conclusions: In line with recent reports from clinical trial data, our data support the use of population genotypic tropism testing as a tool for tropism determination before the start of maraviroc.
Figures
Similar articles
-
Deep-Sequencing Analysis of the Dynamics of HIV-1 Quasiespecies in Naive Patients during a Short Exposure to Maraviroc.J Virol. 2018 May 14;92(11):e00390-18. doi: 10.1128/JVI.00390-18. Print 2018 Jun 1. J Virol. 2018. PMID: 29563289 Free PMC article. Clinical Trial.
-
A genotypic test for HIV-1 tropism combining Sanger sequencing with ultradeep sequencing predicts virologic response in treatment-experienced patients.PLoS One. 2012;7(9):e46334. doi: 10.1371/journal.pone.0046334. Epub 2012 Sep 27. PLoS One. 2012. PMID: 23029482 Free PMC article. Clinical Trial.
-
Evaluation of the genotypic prediction of HIV-1 coreceptor use versus a phenotypic assay and correlation with the virological response to maraviroc: the ANRS GenoTropism study.Antimicrob Agents Chemother. 2010 Aug;54(8):3335-40. doi: 10.1128/AAC.00148-10. Epub 2010 Jun 7. Antimicrob Agents Chemother. 2010. PMID: 20530226 Free PMC article.
-
Genotypic determination of HIV tropism - clinical and methodological recommendations to guide the therapeutic use of CCR5 antagonists.AIDS Rev. 2010 Jul-Sep;12(3):135-48. AIDS Rev. 2010. PMID: 20842202 Review.
-
Effect of HIV-1 subtype and tropism on treatment with chemokine coreceptor entry inhibitors; overview of viral entry inhibition.Crit Rev Microbiol. 2015;41(4):473-87. doi: 10.3109/1040841X.2013.867829. Epub 2014 Mar 17. Crit Rev Microbiol. 2015. PMID: 24635642 Review.
Cited by
-
Correlation of the virological response to short-term maraviroc monotherapy with standard and deep-sequencing-based genotypic tropism prediction methods.Antimicrob Agents Chemother. 2012 Mar;56(3):1202-7. doi: 10.1128/AAC.05857-11. Epub 2011 Dec 5. Antimicrob Agents Chemother. 2012. PMID: 22143533 Free PMC article.
-
Genotypic tropism testing by massively parallel sequencing: qualitative and quantitative analysis.BMC Med Inform Decis Mak. 2011 May 13;11:30. doi: 10.1186/1472-6947-11-30. BMC Med Inform Decis Mak. 2011. PMID: 21569501 Free PMC article.
-
HIV-1 Coreceptor Usage Assessment by Ultra-Deep Pyrosequencing and Response to Maraviroc.PLoS One. 2015 Jun 11;10(6):e0127816. doi: 10.1371/journal.pone.0127816. eCollection 2015. PLoS One. 2015. PMID: 26068869 Free PMC article.
-
Clonal analysis of HIV-1 genotype and function associated with virologic failure in treatment-experienced persons receiving maraviroc: Results from the MOTIVATE phase 3 randomized, placebo-controlled trials.PLoS One. 2018 Dec 26;13(12):e0204099. doi: 10.1371/journal.pone.0204099. eCollection 2018. PLoS One. 2018. PMID: 30586365 Free PMC article. Clinical Trial.
-
Molecular epidemiology of HIV in two highly endemic areas of northeastern South Africa.Arch Virol. 2012 Mar;157(3):455-65. doi: 10.1007/s00705-011-1180-z. Epub 2011 Dec 22. Arch Virol. 2012. PMID: 22189822 Free PMC article.
References
-
- Llibre JM, Schapiro JM, Clotet B. Clinical implications of genotypic resistance to the newer antiretroviral drugs in HIV-1-infected patients with virological failure. Clin Infect Dis. 2010;50:872–81. doi:10.1086/650732. - DOI - PubMed
-
- Feeney Eoin R, Mallon Patrick WG. Impact of mitochondrial toxicity of HIV-1 antiretroviral drugs on lipodystrophy and metabolic dysregulation. Curr Pharm Des. 2010 in press. - PubMed
-
- Lang S, Mary-Krause M, Cotte L, et al. Impact of individual antiretroviral drugs on the risk of myocardial infarction in human immunodeficiency virus-infected patients: a case-control study nested within the French Hospital Database on HIV ANRS cohort CO4. Arch Intern Med. 2010;170:1228–38. doi:10.1001/archinternmed.2010.197. - DOI - PubMed
-
- Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–35. doi:10.1056/NEJMoa062744. - DOI - PubMed
-
- Sabin CA, Worm SW, Weber R, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet. 2008;371:1417–26. doi:10.1016/S0140-6736(08)60423-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous