Preemptive versus postoperative lumiracoxib for analgesia in ambulatory arthroscopic knee surgery
- PMID: 21197285
- PMCID: PMC3004612
- DOI: 10.2147/jpr.s3928
Preemptive versus postoperative lumiracoxib for analgesia in ambulatory arthroscopic knee surgery
Abstract
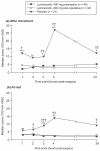
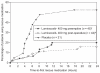
We compared the efficacy and safety of preemptive vs postoperative dosing of lumiracoxib 400 mg in patients undergoing minor ambulatory arthroscopic knee surgery. Eligible patients were randomized to preemptive lumiracoxib, postoperative lumiracoxib, and placebo. The main efficacy parameter was pain intensity (PI) (0-100 mm visual analog scale) in the target knee upon movement, 2 hours after surgery. Other efficacy variables included PI in the target knee at rest and upon movement at 1, 3, 4, and 24 hours, time to first rescue medication intake. In the lumiracoxib preemptive and postoperative groups, the estimated treatment difference compared to placebo for primary endpoint was -4.0 (95% CI: -9, -1; p = 0.007) and -3.5 (95% CI: -8.5, 0; p = 0.052), respectively. There was no statistical significant difference between two active treatment groups (p = 0.602). Both preemptive and postoperative lumiracoxib resulted in significantly lower PI scores at rest and after movement at all time-points and no statistically significant difference was observed between the active treatments. Time to rescue medication intake was comparable for both active treatments. The proportion of adverse events was similar among all groups. We conclude that the efficacy of lumiracoxib 400 mg is not affected by the timing of administration (preemptive or postoperative).
Keywords: NSAIDs; arthroscopic knee surgery; arthroscopy; lumiracoxib; postoperative pain; preemptive dosing.
Figures

References
-
- Buvanendran A, Kroin JS, Berger RA, et al. Upregulation of prostaglandin E2 and interleukins in the central nervous system and peripheral tissue during and after surgery in humans. Anesthesiology. 2006;104:403–10. - PubMed
-
- Buvanendran A, Kroin JS, Tuman KJ, et al. Effects of perioperative administration of a selective cyclooxygenase 2 inhibitor on pain management and recovery of function after knee replacement: a randomized controlled trial. JAMA. 2003;290:2411–8. - PubMed
-
- Chan VW, Clark AJ, Davis JC, et al. The post-operative analgesic efficacy and tolerability of lumiracoxib compared with placebo and naproxen after total knee or hip arthroplasty. Acta Anaesthesiol Scand. 2005;49:1491–500. - PubMed
-
- Daniels S, Gitton X, Zhou W, et al. Efficacy and tolerability of lumiracoxib 200 mg once daily for treatment of primary dysmenorrhea: results from two randomized controlled trials. J Womens Health. 2008;17:423–37. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

