Gustatory and reward brain circuits in the control of food intake
- PMID: 21197607
- PMCID: PMC3434955
- DOI: 10.1007/978-3-7091-0179-7_3
Gustatory and reward brain circuits in the control of food intake
Abstract
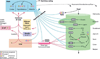
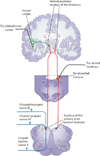
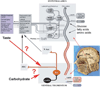
Gustation is a multisensory process allowing for the selection of nutrients and the rejection of irritating and/or toxic compounds. Since obesity is a highly prevalent condition that is critically dependent on food intake and energy expenditure, a deeper understanding of gustatory processing is an important objective in biomedical research. Recent findings have provided evidence that central gustatory processes are distributed across several cortical and subcortical brain areas. Furthermore, these gustatory sensory circuits are closely related to the circuits that process reward. Here, we present an overview of the activation and connectivity between central gustatory and reward areas. Moreover, and given the limitations in number and effectiveness of treatments currently available for overweight patients, we discuss the possibility of modulating neuronal activity in these circuits as an alternative in the treatment of obesity.
Figures

References
-
- Abbott CR, Monteiro M, et al. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044(1):127–131. - PubMed
-
- Altschuler SM, Bao XM, et al. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol. 1989;283(2):248–268. - PubMed
-
- Anand BK, Brobeck JR. Localization of a "feeding center" in the hypothalamus of the rat. Proc Soc Exp Biol Med. 1951;77(2):323–324. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
