Mechanism of Vibrio cholerae autoinducer-1 biosynthesis
- PMID: 21197957
- PMCID: PMC3077805
- DOI: 10.1021/cb1003652
Mechanism of Vibrio cholerae autoinducer-1 biosynthesis
Abstract
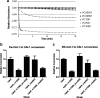
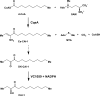
Vibrio cholerae, the causative agent of the disease cholera, uses a cell to cell communication process called quorum sensing to control biofilm formation and virulence factor production. The major V. cholerae quorum-sensing signal CAI-1 has been identified as (S)-3-hydroxytridecan-4-one, and the CqsA protein is required for CAI-1 production. However, the biosynthetic route to CAI-1 remains unclear. Here we report that (S)-adenosylmethionine (SAM) is one of the two biosynthetic substrates for CqsA. CqsA couples SAM and decanoyl-coenzyme A to produce a previously unknown but potent quorum-sensing molecule, 3-aminotridec-2-en-4-one (Ea-CAI-1). The CqsA mechanism is unique; it combines two enzymatic transformations, a β,γ-elimination of SAM and an acyltransferase reaction into a single PLP-dependent catalytic process. Ea-CAI-1 is subsequently converted to CAI-1, presumably through the intermediate tridecane-3,4-dione (DK-CAI-1). We propose that the Ea-CAI-1 to DK-CAI-1 conversion occurs spontaneously, and we identify the enzyme responsible for the subsequent step: conversion of DK-CAI-1 into CAI-1. SAM is the substrate for the synthesis of at least three different classes of quorum-sensing signal molecules, indicating that bacteria have evolved a strategy to leverage an abundant substrate for multiple signaling purposes.
Figures


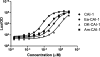



Similar articles
-
The Vibrio cholerae quorum-sensing autoinducer CAI-1: analysis of the biosynthetic enzyme CqsA.Nat Chem Biol. 2009 Dec;5(12):891-5. doi: 10.1038/nchembio.237. Epub 2009 Oct 18. Nat Chem Biol. 2009. PMID: 19838203 Free PMC article.
-
Signal production and detection specificity in Vibrio CqsA/CqsS quorum-sensing systems.Mol Microbiol. 2011 Mar;79(6):1407-17. doi: 10.1111/j.1365-2958.2011.07548.x. Epub 2011 Jan 26. Mol Microbiol. 2011. PMID: 21219472 Free PMC article.
-
Synthesis and Evaluation of Indole-Based Autoinducers on Quorum Sensing in Vibrio cholerae.ACS Infect Dis. 2020 Apr 10;6(4):572-576. doi: 10.1021/acsinfecdis.9b00409. Epub 2020 Mar 23. ACS Infect Dis. 2020. PMID: 32182033
-
Bacterial gene regulation by alpha-hydroxyketone signaling.Trends Microbiol. 2010 Jul;18(7):288-97. doi: 10.1016/j.tim.2010.03.004. Epub 2010 Apr 8. Trends Microbiol. 2010. PMID: 20382022 Review.
-
α-Hydroxyketone synthesis and sensing by Legionella and Vibrio.Sensors (Basel). 2012;12(3):2899-919. doi: 10.3390/s120302899. Epub 2012 Mar 2. Sensors (Basel). 2012. PMID: 22736983 Free PMC article. Review.
Cited by
-
Broad spectrum pro-quorum-sensing molecules as inhibitors of virulence in vibrios.PLoS Pathog. 2012;8(6):e1002767. doi: 10.1371/journal.ppat.1002767. Epub 2012 Jun 28. PLoS Pathog. 2012. PMID: 22761573 Free PMC article.
-
Noncanonical Functions of Enzyme Cofactors as Building Blocks in Natural Product Biosynthesis.JACS Au. 2022 Aug 17;2(9):1950-1963. doi: 10.1021/jacsau.2c00391. eCollection 2022 Sep 26. JACS Au. 2022. PMID: 36186570 Free PMC article. Review.
-
S-Adenosylmethionine: more than just a methyl donor.Nat Prod Rep. 2023 Sep 20;40(9):1521-1549. doi: 10.1039/d2np00086e. Nat Prod Rep. 2023. PMID: 36891755 Free PMC article. Review.
-
Parallel quorum-sensing system in Vibrio cholerae prevents signal interference inside the host.PLoS Pathog. 2020 Feb 14;16(2):e1008313. doi: 10.1371/journal.ppat.1008313. eCollection 2020 Feb. PLoS Pathog. 2020. PMID: 32059031 Free PMC article.
-
The Interface of Vibrio cholerae and the Gut Microbiome.Gut Microbes. 2021 Jan-Dec;13(1):1937015. doi: 10.1080/19490976.2021.1937015. Gut Microbes. 2021. PMID: 34180341 Free PMC article. Review.
References
-
- Miller M. B.; Bassler B. L. (2001) Quorum sensing in bacteria. Ann. Rev. Microbiol. 55, 165–199. - PubMed
-
- Sun W.; Cao J. G.; Teng K.; Meighen E. A. (1994) Biosynthesis of poly-3-hydroxybutyrate in the luminescent bacterium, Vibrio harveyi, and regulation by the lux autoinducer, N-(3-hydroxybutanoyl)homoserine lactone. J. Biol. Chem. 269, 20785–20790. - PubMed
-
- More M. I.; Finger L. D.; Stryker J. L.; Fuqua C.; Eberhard A.; Winans S. C. (1996) Enzymatic synthesis of a quorum-sensing autoinducer through use of defined substrates. Science 272, 1655–1658. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

