Fibrin acts as biomimetic niche inducing both differentiation and stem cell marker expression of early human endothelial progenitor cells
- PMID: 21199008
- PMCID: PMC6496210
- DOI: 10.1111/j.1365-2184.2010.00715.x
Fibrin acts as biomimetic niche inducing both differentiation and stem cell marker expression of early human endothelial progenitor cells
Abstract
Objectives: Transplantation of endothelial progenitor cells (EPCs) is a promising approach for revascularization of tissue. We have used a natural and biocompatible biopolymer, fibrin, to induce cell population growth, differentiation and functional activity of EPCs.
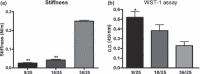
Materials and methods: Peripheral blood mononuclear cells were cultured for 1 week to obtain early EPCs. Fibrin was characterized for stiffness and capability to sustain cell population expansion at different fibrinogen-thrombin ratios. Viability, differentiation and angiogenic properties of EPCs were evaluated and compared to those of EPCs grown on fibronectin.
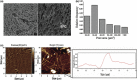
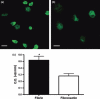
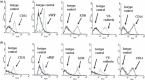
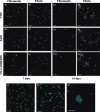
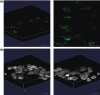
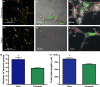
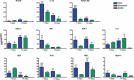
Results: Fibrin had a nanometric fibrous structure forming a porous network. Fibrinogen concentration significantly influenced fibrin stiffness and cell growth: 9 mg/ml fibrinogen and 25 U/ml thrombin was the best ratio for enhanced cell viability. Moreover, cell viability was significantly higher on fibrin compared to being on fibronectin. Even though no significant difference was observed in expression of endothelial markers, culture on fibrin elicited marked induction of stem cell markers OCT 3/4 and NANOG. In vitro angiogenesis assay on Matrigel showed that EPCs grown on fibrin retain angiogenetic capability as EPCs grown on fibronectin, but significantly better release of cytokines involved in cell recruitment was produced by EPC grown on fibrin.
Conclusion: Fibrin is a suitable matrix for EPC growth, differentiation and angiogenesis capability, suggesting that fibrin gel may be very useful for regenerative medicine.
© 2010 Blackwell Publishing Ltd.
Figures

References
-
- Hamdi H, Furuta A, Bellamy V, Bel A, Puymirat E, Peyrard S et al. (2009) Cell delivery: intramyocardial injections or epicardial deposition? A head‐to‐head comparison Ann. Thorac. Surg. 87, 1196–1203. - PubMed
-
- Shaikh FM, Callanan A, Kavanagh EG, Burke PE, Grace PA, McGloughlin TM (2008) Fibrin: a natural biodegradable scaffold in vascular tissue engineering. Cells Tissues Organs 188, 333–346. - PubMed
-
- Nakatsu MN, Sainson RC, Aoto JN, Taylor KL, Aitkenhead M, Perez‐del‐Pulgar S et al. (2003) Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin‐1. Microvasc. Res. 66, 102–112. - PubMed
-
- Cox S, Cole M, Tawil B (2004) Behavior of human dermal fibroblasts in three‐dimensional fibrin clots: dependence on fibrinogen and thrombin concentration. Tissue Eng. 10, 942–954. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

