Caveolin-1 is required for contractile phenotype expression by airway smooth muscle cells
- PMID: 21199324
- PMCID: PMC3822954
- DOI: 10.1111/j.1582-4934.2010.01246.x
Caveolin-1 is required for contractile phenotype expression by airway smooth muscle cells
Abstract
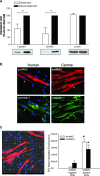
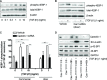
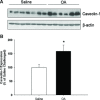
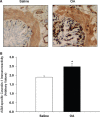
Airway smooth muscle cells exhibit phenotype plasticity that underpins their ability to contribute both to acute bronchospasm and to the features of airway remodelling in chronic asthma. A feature of mature, contractile smooth muscle cells is the presence of abundant caveolae, plasma membrane invaginations that develop from the association of lipid rafts with caveolin-1, but the functional role of caveolae and caveolin-1 in smooth muscle phenotype plasticity is unknown. Here, we report a key role for caveolin-1 in promoting phenotype maturation of differentiated airway smooth muscle induced by transforming growth factor (TGF)-β(1). As assessed by Western analysis and laser scanning cytometry, caveolin-1 protein expression was selectively enriched in contractile phenotype airway myocytes. Treatment with TGF-β(1) induced profound increases in the contractile phenotype markers sm-α-actin and calponin in cells that also accumulated abundant caveolin-1; however, siRNA or shRNAi inhibition of caveolin-1 expression largely prevented the induction of these contractile phenotype marker proteins by TGF-β(1). The failure by TGF-β(1) to adequately induce the expression of these smooth muscle specific proteins was accompanied by a strongly impaired induction of eukaryotic initiation factor-4E binding protein(4E-BP)1 phosphorylation with caveolin-1 knockdown, indicating that caveolin-1 expression promotes TGF-β(1) signalling associated with myocyte maturation and hypertrophy. Furthermore, we observed increased expression of caveolin-1 within the airway smooth muscle bundle of guinea pigs repeatedly challenged with allergen, which was associated with increased contractile protein expression, thus providing in vivo evidence linking caveolin-1 expression with accumulation of contractile phenotype myocytes. Collectively, we identify a new function for caveolin-1 in controlling smooth muscle phenotype; this mechanism could contribute to allergic asthma.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures


References
-
- Halayko AJ, Solway J. Molecular mechanisms of phenotypic plasticity in smooth muscle cells. J Appl Physiol. 2001;90:358–68. - PubMed
-
- Halayko AJ, Tran T, Ji SY, et al. Airway smooth muscle phenotype and function: interactions with current asthma therapies. Curr Drug Targets. 2006;7:525–40. - PubMed
-
- Halayko AJ, Tran T, Gosens R. Phenotype and functional plasticity of airway smooth muscle: role of caveolae and caveolins. Proc Am Thorac Soc. 2008;5:80–8. - PubMed
-
- Darby PJ, Kwan CY, Daniel EE. Caveolae from canine airway smooth muscle contain the necessary components for a role in Ca(2+) handling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1226–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

