Regulation of blood flow in the microcirculation: role of conducted vasodilation
- PMID: 21199397
- PMCID: PMC3115483
- DOI: 10.1111/j.1748-1716.2010.02244.x
Regulation of blood flow in the microcirculation: role of conducted vasodilation
Abstract
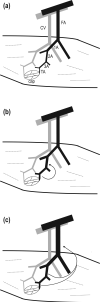
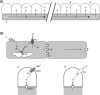
This review is concerned with understanding how vasodilation initiated from local sites in the tissue can spread to encompass multiple branches of the resistance vasculature. Within tissues, arteriolar networks control the distribution and magnitude of capillary perfusion. Vasodilation arising from the microcirculation can 'ascend' into feed arteries that control blood flow into arteriolar networks. Thus distal segments of the resistance network signal proximal segments to dilate and thereby increase total oxygen supply to parenchymal cells. August Krogh proposed that innervation of capillaries provided the mechanism for a spreading vasodilatory response. With greater understanding of the ultrastructural organization of resistance networks, an alternative explanation has emerged: Electrical signalling from cell to cell along the vessel wall through gap junctions. Hyperpolarization originates from ion channel activation at the site of stimulation with the endothelium serving as the predominant cellular pathway for signal conduction along the vessel wall. As hyperpolarization travels, it is transmitted into surrounding smooth muscle cells through myoendothelial coupling to promote relaxation. Conducted vasodilation (CVD) encompasses greater distances than can be explained by passive decay and understanding such behaviour is the focus of current research efforts. In the context of athletic performance, the ability of vasodilation to ascend into feed arteries is essential to achieving peak levels of muscle blood flow. CVD is tempered by sympathetic neuroeffector signalling when governing muscle blood flow at rest and during exercise. Impairment of conduction during ageing and in diseased states can limit physical work capacity by restricting muscle blood flow.
© 2011 The Authors. Acta Physiologica © 2011 Scandinavian Physiological Society.
Figures
References
-
- Bartlett IS, Segal SS. Resolution of smooth muscle and endothelial pathways for conduction along hamster cheek pouch arterioles. Am J Physiol Heart Circ Physiol. 2000;278:H604–H612. - PubMed
-
- Beach JM, McGahren ED, Duling BR. Capillaries and arterioles are electrically coupled in hamster cheek pouch. Am J Physiol Heart Circ Physiol. 1998;275:H1489–H1496. - PubMed
-
- Bearden SE, Linn E, Ashley BS, Looft-Wilson RC. Age-related changes in conducted vasodilation: effects of exercise training and role in functional hyperemia. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1717–R1721. - PubMed
-
- Beny JL. Information Networks in the Arterial Wall. News Physiol Sci. 1999;14:68–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

