Inactivation of Brca2 promotes Trp53-associated but inhibits KrasG12D-dependent pancreatic cancer development in mice
- PMID: 21199651
- PMCID: PMC3066280
- DOI: 10.1053/j.gastro.2010.12.039
Inactivation of Brca2 promotes Trp53-associated but inhibits KrasG12D-dependent pancreatic cancer development in mice
Abstract
Background & aims: Inherited mutations in the BRCA2 tumor suppressor have been associated with an increased risk of pancreatic cancer. To establish the contribution of Brca2 to pancreatic cancer we developed a mouse model of pancreas-specific Brca2 inactivation. Because BRCA2-inactivating mutations cause defects in repair of DNA double-strand breaks that result in chromosomal instability, we evaluated whether Brca2 inactivation induced instability in pancreatic tissue from these mice and whether associated pancreatic tumors were hypersensitive to DNA damaging agents.
Methods: We developed mouse models that combined pancreas-specific Kras activation and Trp53 deletion with Brca2 inactivation. Development of pancreatic cancer was assessed; tumors and nonmalignant tissues were analyzed for chromosomal instability and apoptosis. Cancer cell lines were evaluated for sensitivity to DNA damaging agents.
Results: In the presence of disrupted Trp53, Brca2 inactivation promoted the development of premalignant lesions and pancreatic tumors that reflected the histology of human disease. Cancer cell lines derived from these tumors were hypersensitive to specific DNA damaging agents. In contrast, in the presence of KrasG12D, Brca2 inactivation promoted chromosomal instability and apoptosis and unexpectedly inhibited growth of premalignant lesions and tumors.
Conclusions: Trp53 signaling must be modified before inactivation of the Brca2 wild-type allele, irrespective of Kras status, for Brca2-deficient cells to form tumors.
Copyright © 2011 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

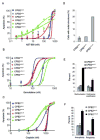



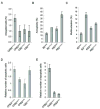

Comment in
-
Timing is everything: Brca2 and p53 mutations in pancreatic cancer.Gastroenterology. 2011 Apr;140(4):1143-6. doi: 10.1053/j.gastro.2011.02.026. Epub 2011 Feb 23. Gastroenterology. 2011. PMID: 21352873 No abstract available.
References
-
- Moynahan ME, Pierce AJ, Jasin M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell. 2001;7:263–72. - PubMed
-
- Consortium TBCL. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91:1310–6. - PubMed
-
- Couch FJ, Johnson MR, Rabe K, et al. Germ line Fanconi anemia complementation group C mutations and pancreatic cancer. Cancer Res. 2005;65:383–6. - PubMed
-
- Murphy KM, Brune KA, Griffin C, et al. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 17% Cancer Res. 2002;62:3789–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

