Snail2 is an essential mediator of Twist1-induced epithelial mesenchymal transition and metastasis
- PMID: 21199805
- PMCID: PMC3025803
- DOI: 10.1158/0008-5472.CAN-10-2330
Snail2 is an essential mediator of Twist1-induced epithelial mesenchymal transition and metastasis
Abstract
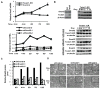
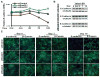
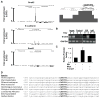
To metastasize, carcinoma cells must attenuate cell-cell adhesion to disseminate into distant organs. A group of transcription factors, including Twist1, Snail1, Snail2, ZEB1, and ZEB2, have been shown to induce epithelial mesenchymal transition (EMT), thus promoting tumor dissemination. However, it is unknown whether these transcription factors function independently or coordinately to activate the EMT program. Here we report that direct induction of Snail2 is essential for Twist1 to induce EMT. Snail2 knockdown completely blocks the ability of Twist1 to suppress E-cadherin transcription. Twist1 binds to an evolutionarily conserved E-box on the proximate Snail2 promoter to induce its transcription. Snail2 induction is essential for Twist1-induced cell invasion and distant metastasis in mice. In human breast tumors, the expression of Twist1 and Snail2 is highly correlated. Together, our results show that Twist1 needs to induce Snail2 to suppress the epithelial branch of the EMT program and that Twist1 and Snail2 act together to promote EMT and tumor metastasis.
© 2011 AACR.
Conflict of interest statement
No potential conflict of interest was disclosed.
Figures



References
-
- Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. - PubMed
-
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. - PubMed
-
- Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–29. - PubMed
-
- Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

