The Ca2+-dependent phosphatase calcineurin controls the formation of the Carma1-Bcl10-Malt1 complex during T cell receptor-induced NF-kappaB activation
- PMID: 21199863
- PMCID: PMC3045007
- DOI: 10.1074/jbc.M110.155895
The Ca2+-dependent phosphatase calcineurin controls the formation of the Carma1-Bcl10-Malt1 complex during T cell receptor-induced NF-kappaB activation
Abstract
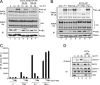
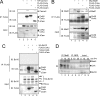
T cell receptor (TCR) ligation induces increased diacylglycerol and Ca(2+) levels in T cells, and both secondary messengers are crucial for TCR-induced nuclear factor of activated T cells (NF-AT) and NF-κB signaling pathways. One prominent calcium-dependent enzyme involved in the regulation of NF-AT and NF-κB signaling pathways is the protein phosphatase calcineurin. However, in contrast to NF-AT, which is directly dephosphorylated by calcineurin, the molecular basis of the calcium-calcineurin dependence of the TCR-induced NF-κB activity remains largely unknown. Here, we demonstrate that calcineurin regulates TCR-induced NF-κB activity by controlling the formation of a protein complex composed of Carma1, Bcl10, and Malt1 (CBM complex). For instance, increased calcium levels induced by ionomycin or thapsigargin augmented the phorbol 12-myristate 13-acetate-induced formation of the CBM complex and activation of NF-κB, whereas removal of calcium by the calcium chelator EGTA-acetoxymethyl ester (AM) attenuated both processes. Furthermore, inhibition of the calcium-dependent phosphatase calcineurin with the immunosuppressive agent cyclosporin A (CsA) or FK506 as well as siRNA-mediated knockdown of calcineurin A strongly affected the PMA + ionomycin- or anti-CD3 + CD28-induced CBM complex assembly. Mechanistically, the positive effect of calcineurin on the CBM complex formation seems to be linked to a dephosphorylation of Bcl10. For instance, Bcl10 was found to be hyperphosphorylated in Jurkat T cells upon treatment with CsA or EGTA-AM, and calcineurin dephosphorylated Bcl10 in vivo and in vitro. Furthermore, we show here that calcineurin A interacts with the CBM complex. In summary, the evidence provided here argues for a previously unanticipated role of calcineurin in CBM complex formation as a molecular basis of the inhibitory function of CsA or FK506 on TCR-induced NF-κB activity.
Figures







References
-
- Marienfeld R., Neumann M., Chuvpilo S., Escher C., Kneitz B., Avots A., Schimpl A., Serfling E. (1997) Eur. J. Immunol. 27, 1601–1609 - PubMed
-
- Trushin S. A., Pennington K. N., Algeciras-Schimnich A., Paya C. V. (1999) J. Biol. Chem. 274, 22923–22931 - PubMed
-
- Thome M., Weil R. (2007) Trends Immunol. 28, 281–288 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

