Conserved intramolecular disulfide bond is critical to trafficking and fate of ATP-binding cassette (ABC) transporters ABCB6 and sulfonylurea receptor 1 (SUR1)/ABCC8
- PMID: 21199866
- PMCID: PMC3048732
- DOI: 10.1074/jbc.M110.174516
Conserved intramolecular disulfide bond is critical to trafficking and fate of ATP-binding cassette (ABC) transporters ABCB6 and sulfonylurea receptor 1 (SUR1)/ABCC8
Abstract
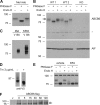
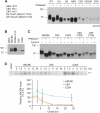
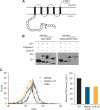
The ATP-binding cassette (ABC) transporter ABCB6 is a mitochondrial porphyrin transporter that activates porphyrin biosynthesis. ABCB6 lacks a canonical mitochondrial targeting sequence but reportedly traffics to other cellular compartments such as the plasma membrane. How ABCB6 reaches these destinations is unknown. In this study, we show that endogenous ABCB6 is glycosylated in multiple cell types, indicating trafficking through the endoplasmic reticulum (ER), and has only one atypical site for glycosylation (NXC) in its amino terminus. ABCB6 remained glycosylated when the highly conserved cysteine (Cys-8) was substituted with serine to make a consensus site, NXS. However, this substitution blocked ER exit and produced ABCB6 degradation, which was mostly reversed by the proteasomal inhibitor MG132. The amino terminus of ABCB6 has an additional highly conserved ER luminal cysteine (Cys-26). When Cys-26 was mutated alone or in combination with Cys-8, it also resulted in instability and ER retention. Further analysis revealed that these two cysteines form a disulfide bond. We discovered that other ABC transporters with an amino terminus in the ER had similarly configured conserved cysteines. This analysis led to the discovery of a disease-causing mutation in the sulfonylurea receptor 1 (SUR1)/ABCC8 from a patient with hyperinsulinemic hypoglycemia. The mutant allele only contains a mutation in a conserved amino-terminal cysteine, producing SUR1 that fails to reach the cell surface. These results suggest that for ABC transporters the propensity to form a disulfide bond in the ER defines a unique checkpoint that determines whether a protein is ER-retained.
Figures




Similar articles
-
Regulation of KATP channel expression and activity by the SUR1 nucleotide binding fold 1.Channels (Austin). 2007 Jul-Aug;1(4):315-23. doi: 10.4161/chan.5083. Epub 2007 Sep 25. Channels (Austin). 2007. PMID: 18708750
-
Identification of a familial hyperinsulinism-causing mutation in the sulfonylurea receptor 1 that prevents normal trafficking and function of KATP channels.J Biol Chem. 2002 May 10;277(19):17139-46. doi: 10.1074/jbc.M200363200. Epub 2002 Feb 26. J Biol Chem. 2002. PMID: 11867634
-
Defective trafficking and function of KATP channels caused by a sulfonylurea receptor 1 mutation associated with persistent hyperinsulinemic hypoglycemia of infancy.Proc Natl Acad Sci U S A. 2001 Feb 27;98(5):2882-7. doi: 10.1073/pnas.051499698. Proc Natl Acad Sci U S A. 2001. PMID: 11226335 Free PMC article.
-
Quality control of human ABCG2 protein in the endoplasmic reticulum: ubiquitination and proteasomal degradation.Adv Drug Deliv Rev. 2009 Jan 31;61(1):66-72. doi: 10.1016/j.addr.2008.08.008. Epub 2008 Dec 11. Adv Drug Deliv Rev. 2009. PMID: 19111842 Review.
-
Persistent hyperinsulinemic hypoglycemia of infancy.Am J Med Genet A. 2003 Nov 1;122A(4):351-3. doi: 10.1002/ajmg.a.20480. Am J Med Genet A. 2003. PMID: 14518075 Review.
Cited by
-
Efficient purification and reconstitution of ATP binding cassette transporter B6 (ABCB6) for functional and structural studies.J Biol Chem. 2013 Aug 2;288(31):22658-69. doi: 10.1074/jbc.M113.485284. Epub 2013 Jun 21. J Biol Chem. 2013. PMID: 23792964 Free PMC article.
-
Role of cysteine residues in cell surface expression of the human riboflavin transporter-2 (hRFT2) in intestinal epithelial cells.Am J Physiol Gastrointest Liver Physiol. 2011 Jul;301(1):G100-9. doi: 10.1152/ajpgi.00120.2011. Epub 2011 Apr 21. Am J Physiol Gastrointest Liver Physiol. 2011. PMID: 21512156 Free PMC article.
-
ABCB6, an ABC Transporter Impacting Drug Response and Disease.AAPS J. 2017 Nov 30;20(1):8. doi: 10.1208/s12248-017-0165-6. AAPS J. 2017. PMID: 29192381 Free PMC article. Review.
-
The role of ATP-binding Cassette subfamily B member 6 in the inner ear.Nat Commun. 2024 Nov 18;15(1):9885. doi: 10.1038/s41467-024-53663-x. Nat Commun. 2024. PMID: 39557842 Free PMC article.
-
Role of Derlin-1 protein in proteostasis regulation of ATP-sensitive potassium channels.J Biol Chem. 2012 Mar 23;287(13):10482-10493. doi: 10.1074/jbc.M111.312223. Epub 2012 Feb 6. J Biol Chem. 2012. PMID: 22311976 Free PMC article.
References
-
- Dean M. (2005) Methods Enzymol. 400, 409–429 - PubMed
-
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L., Drumm M. L., Iannuzzi M. C., Collins F. S., Tsui L. (1989) Science 245, 1066–1073 - PubMed
-
- Cheng S. H., Gregory R. J., Marshall J., Paul S., Souza D. W., White G. A., O'Riordan C. R., Smith A. E. (1990) Cell 63, 827–834 - PubMed
-
- Ellgaard L., Molinari M., Helenius A. (1999) Science 286, 1882–1888 - PubMed
-
- Ellgaard L., Helenius A. (2003) Nat. Rev. Mol. Cell Biol. 4, 181–191 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

