Shiga toxin subtypes display dramatic differences in potency
- PMID: 21199911
- PMCID: PMC3067513
- DOI: 10.1128/IAI.01182-10
Shiga toxin subtypes display dramatic differences in potency
Abstract
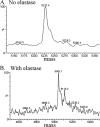
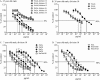
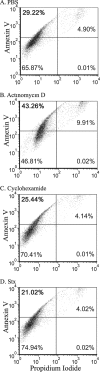
Purified Shiga toxin (Stx) alone is capable of producing systemic complications, including hemolytic-uremic syndrome (HUS), in animal models of disease. Stx includes two major antigenic forms (Stx1 and Stx2), with minor variants of Stx2 (Stx2a to -h). Stx2a is more potent than Stx1. Epidemiologic studies suggest that Stx2 subtypes also differ in potency, but these differences have not been well documented for purified toxin. The relative potencies of five purified Stx2 subtypes, Stx2a, Stx2b, Stx2c, Stx2d, and activated (elastase-cleaved) Stx2d, were studied in vitro by examining protein synthesis inhibition using Vero monkey kidney cells and inhibition of metabolic activity (reduction of resazurin to fluorescent resorufin) using primary human renal proximal tubule epithelial cells (RPTECs). In both RPTECs and Vero cells, Stx2a, Stx2d, and elastase-cleaved Stx2d were at least 25 times more potent than Stx2b and Stx2c. In vivo potency in mice was also assessed. Stx2b and Stx2c had potencies similar to that of Stx1, while Stx2a, Stx2d, and elastase-cleaved Stx2d were 40 to 400 times more potent than Stx1.
Figures



Similar articles
-
Switching Shiga Toxin (Stx) Type from Stx2d to Stx2a but Not Stx2c Alters Virulence of Stx-Producing Escherichia coli (STEC) Strain B2F1 in Streptomycin (Str)-Treated Mice.Toxins (Basel). 2021 Jan 15;13(1):64. doi: 10.3390/toxins13010064. Toxins (Basel). 2021. PMID: 33467588 Free PMC article.
-
Reduced Toxicity of Shiga Toxin (Stx) Type 2c in Mice Compared to Stx2d Is Associated with Instability of Stx2c Holotoxin.Toxins (Basel). 2015 Jun 23;7(6):2306-20. doi: 10.3390/toxins7062306. Toxins (Basel). 2015. PMID: 26110507 Free PMC article.
-
Shiga Toxin Subtypes of Non-O157 Escherichia coli Serogroups Isolated from Cattle Feces.Front Cell Infect Microbiol. 2017 Apr 11;7:121. doi: 10.3389/fcimb.2017.00121. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28443248 Free PMC article.
-
New aspects in the pathogenesis of enteropathic hemolytic uremic syndrome.Semin Thromb Hemost. 2006 Mar;32(2):105-12. doi: 10.1055/s-2006-939766. Semin Thromb Hemost. 2006. PMID: 16575685 Review.
-
Escherichia coli Shiga toxin.J Nat Toxins. 2000 Aug;9(3):299-313. J Nat Toxins. 2000. PMID: 10994531 Review.
Cited by
-
Shiga Toxin (Stx) Classification, Structure, and Function.Microbiol Spectr. 2014 Aug;2(4):EHEC-0024-2013. doi: 10.1128/microbiolspec.EHEC-0024-2013. Microbiol Spectr. 2014. PMID: 25530917 Free PMC article. Review.
-
The microcosm mediates the persistence of shiga toxin-producing Escherichia coli in freshwater ecosystems.Appl Environ Microbiol. 2013 Aug;79(16):4821-8. doi: 10.1128/AEM.01281-13. Epub 2013 Jun 7. Appl Environ Microbiol. 2013. PMID: 23747699 Free PMC article.
-
Characterization of Shiga Toxin Subtypes and Virulence Genes in Porcine Shiga Toxin-Producing Escherichia coli.Front Microbiol. 2016 Apr 21;7:574. doi: 10.3389/fmicb.2016.00574. eCollection 2016. Front Microbiol. 2016. PMID: 27148249 Free PMC article.
-
Virulence Potential of Escherichia coli Isolates from a Beef Farm in Puerto Rico.Microbiol Resour Announc. 2022 Nov 17;11(11):e0044322. doi: 10.1128/mra.00443-22. Epub 2022 Oct 26. Microbiol Resour Announc. 2022. PMID: 36286991 Free PMC article.
-
Bacteriophages of Shiga Toxin-Producing Escherichia coli and Their Contribution to Pathogenicity.Pathogens. 2021 Mar 29;10(4):404. doi: 10.3390/pathogens10040404. Pathogens. 2021. PMID: 33805526 Free PMC article. Review.
References
-
- Bielaszewska, M., A. W. Friedrich, T. Aldick, R. Schürk-Bulgrin, and H. Karch. 2006. Shiga toxin activatable by intestinal mucus in Escherichia coli isolated from humans: predictor for a severe clinical outcome. Clin. Infect. Dis. 43:1160-1167. - PubMed
-
- Carter, A. O., et al. 1987. A severe outbreak of Escherichia coli O157:H7-associated hemorrhagic colitis in a nursing home. N. Engl. J. Med. 317:1496-1500. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

