Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma
- PMID: 21199938
- PMCID: PMC3024691
- DOI: 10.1073/pnas.1013965108
Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma
Abstract
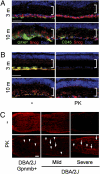
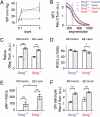
Optic nerve head (ONH) astrocytes have been proposed to play both protective and deleterious roles in glaucoma. We now show that, within the postlaminar ONH myelination transition zone (MTZ), there are astrocytes that normally express Mac-2 (also known as Lgals3 or galectin-3), a gene typically expressed only in phagocytic cells. Surprisingly, even in healthy mice, MTZ and other ONH astrocytes constitutive internalize large axonal evulsions that contain whole organelles. In mouse glaucoma models, MTZ astrocytes further up-regulate Mac-2 expression. During glaucomatous degeneration, there are dystrophic processes in the retina and optic nerve, including the MTZ, which contain protease resistant γ-synuclein. The increased Mac-2 expression by MTZ astrocytes during glaucoma likely depends on this γ-synuclein, as mice lacking γ-synuclein fail to up-regulate Mac-2 at the MTZ after elevation of intraocular pressure. These results suggest the possibility that a newly discovered normal degradative pathway for axons might contribute to glaucomatous neurodegeneration.
Conflict of interest statement
Conflict of interest statement: N.M.-A. and I.S. have a pending patent application on the use of anti-Parkinson disease treatments in glaucoma.
Figures



References
-
- Inman DM, Horner PJ. Reactive nonproliferative gliosis predominates in a chronic mouse model of glaucoma. Glia. 2007;55:942–953. - PubMed
-
- WoldeMussie E, Ruiz G, Wijono M, Wheeler LA. Neuroprotection of retinal ganglion cells by brimonidine in rats with laser-induced chronic ocular hypertension. Invest Ophthalmol Vis Sci. 2001;42:2849–2855. - PubMed
-
- Son JL, et al. Glaucomatous optic nerve injury involves early astrocyte reactivity and late oligodendrocyte loss. Glia. 2010;58:780–789. - PubMed
-
- Hernandez MR, Miao H, Lukas T. Astrocytes in glaucomatous optic neuropathy. Prog Brain Res. 2008;173:353–373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA016602/DA/NIDA NIH HHS/United States
- 075615/Wellcome Trust/United Kingdom
- 5P41RR004050/RR/NCRR NIH HHS/United States
- 5R21EY019737/EY/NEI NIH HHS/United States
- T32 EY017203/EY/NEI NIH HHS/United States
- 075615/Z/04/z/WT_/Wellcome Trust/United Kingdom
- 5R01GM82949/GM/NIGMS NIH HHS/United States
- R01 GM082949/GM/NIGMS NIH HHS/United States
- R01 DA016602/DA/NIDA NIH HHS/United States
- T32 EY007143/EY/NEI NIH HHS/United States
- R01 EY019305/EY/NEI NIH HHS/United States
- 5T32 EY07143-12/EY/NEI NIH HHS/United States
- R21 EY019737/EY/NEI NIH HHS/United States
- P41 RR004050/RR/NCRR NIH HHS/United States
- 5R01EY019305/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

