cis-cis-trans-Bis(acetonitrile-κN)dichloridobis(triphenyl-phosphine-κP)ruthenium(II) acetonitrile disolvate
- PMID: 21200532
- PMCID: PMC2915117
- DOI: 10.1107/S1600536807065968
cis-cis-trans-Bis(acetonitrile-κN)dichloridobis(triphenyl-phosphine-κP)ruthenium(II) acetonitrile disolvate
Abstract
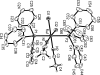
The title compound, [RuCl(2)(C(2)H(3)N)(2)(C(18)H(15)P)(2)]·2C(2)H(3)N, was obtained upon stirring an acetonitrile/ethanol solution of [RuCl(2)(PPh(3))(3)]. In the crystal structure, each Ru(II) ion is coordinated by two Cl [Ru-Cl = 2.4308 (7) and 2.4139 (7) Å], two N [Ru-N = 2.016 (2) and 2.003 (2) Å], and two P [Ru-P = 2.3688 (7) and 2.3887 (7) Å] atoms in a distorted octa-hedral geometry. Packing inter-actions include typical C-H⋯π contacts involving phenyl groups as well as weak hydrogen bonds between CH(3)CN methyl H atoms and Cl or solvent CH(3)CN N atoms.
Figures

References
-
- Bruker (2006). APEX2 (Version 2.0) and SAINT (Version 7.23A). Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Caulton, K. G. (1974). J. Am. Chem. Soc.96, 3005–3006.
-
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
-
- Gilbert, J. D. & Wilkinson, G. (1969). J. Chem. Soc. A, pp. 1749–1753.
-
- Hallman, P. S., Stephenson, T. A. & Wilkinson, G. (1970). Inorg. Synth.12, 237–240.
LinkOut - more resources
Full Text Sources
Miscellaneous
