(+)-(1S,5R,10S)-11,11-Dimeth-yl-4-oxa-tricyclo-[8.4.0.0]tetra-deca-ne-3,12-dione
- PMID: 21200853
- PMCID: PMC2915339
- DOI: 10.1107/S1600536807066159
(+)-(1S,5R,10S)-11,11-Dimeth-yl-4-oxa-tricyclo-[8.4.0.0]tetra-deca-ne-3,12-dione
Abstract
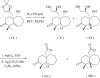
The title compound, C(15)H(22)O(3), was prepared via amino-acid-promoted Robinson annulation followed by tandem Pd/C-mediated hydrogenation and oxidative cyclization. This product was instrumental in determining the feasibility of a stereocontrolled hydrogenation in which the directing hydroxyl group is adjacent to the 6-7-ring network and its olefinic component. The asymmetric unit consists of a single mol-ecule with normal geometric parameters. The absolute configuration was assigned based on the known enanti-omeric prescursor. Inter-molecular C-H⋯O inter-actions link each mol-ecule with four neighboring mol-ecules.
Figures




References
-
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin 2, pp. S1–S19.
-
- Brown, J. M. (1987). Angew. Chem. Int. Ed. Engl.26, 190–203.
-
- Bruker (1999). SHELXTL Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Crabtree, R. H. & Davis, M. W. (1986). J. Org. Chem.51, 2655–2661.
-
- Desiraju, G. R. & Steiner, T. (1999). The Weak Hydrogen Bond in Structural Chemistry and Biology IUCr Monographs on Crystallography, 9. Oxford University Press.
LinkOut - more resources
Full Text Sources
