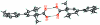2,6-Dimethyl-4-m-tolyl-cyclo-hex-3-enecarboxylic acid
- PMID: 21201085
- PMCID: PMC2959375
- DOI: 10.1107/S1600536808027542
2,6-Dimethyl-4-m-tolyl-cyclo-hex-3-enecarboxylic acid
Abstract
The title compound, C(16)H(20)O(2), was synthesized to study the hydrogen-bonding inter-action of the two enanti-omers in the solid state. The racemate is made up of carboxylic acid RS dimers. Inter-molecular O-H⋯O hydrogen bonds produce centrosymmetric R(2) (2)(8) rings which dimerize the two chiral enanti-omers through their carboxyl groups. The chirality of this compound is generated by the presence of the double bond in the cyclo-hexene ring and a chiral axis due to the meta-methyl substituent on the aromatic ring.
Figures
References
-
- Bernstein, J., Davis, R., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl.34, 1555–1573.
-
- Bruker (2007). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Burla, M. C., Caliandro, R., Camalli, M., Carrozzini, B., Cascarano, G. L., De Caro, L., Giacovazzo, C., Polidori, G. & Spagna, R. (2005). J. Appl. Cryst.38, 381–388.
-
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
-
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. - PubMed
LinkOut - more resources
Full Text Sources