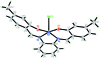
Chlorido{5,5'-dimethyl-2,2'-[1,2-phenyl-enebis(nitrilo-methyl-idyne)]diphenolato-κO,N,N',O'}manganese(III)
- PMID: 21202022
- PMCID: PMC2960927
- DOI: 10.1107/S1600536808007459
Chlorido{5,5'-dimethyl-2,2'-[1,2-phenyl-enebis(nitrilo-methyl-idyne)]diphenolato-κO,N,N',O'}manganese(III)
Abstract
In the title complex, [Mn(C(22)H(18)N(2)O(2))Cl], the Mn(III) center is in a distorted square-pyramidal configuration; the basal plane is formed by the N(2)O(2) donors of the tetra-dentate Schiff base dianion, with the two phenol O atoms and two imine N atoms each mutually cis. The chloride ion occupies the apical coordination site. The dihedral angle between the two outer phenolate rings of the tetra-dentate ligand is 18.24 (9)°. The central benzene ring makes dihedral angles of 13.71 (8) and 30.50 (8)° with the two outer phenolate rings. In the crystal structure, weak C-H⋯Cl inter-actions link the mol-ecules into screw helices along the b direction. These helices are further connected by weak C-H⋯O inter-actions into a three-dimensional network. The crystal structure is further stabilized by C-H⋯π inter-actions involving the central benzene ring.
Figures
Similar articles
-
Chlorido{5,5'-dimeth-oxy-2,2'-[1,2-phenyl-enebis(nitrilo-methyl-idyne)]diphenolato-κO,N,N',O'}manganese(III).Acta Crystallogr Sect E Struct Rep Online. 2008 Apr 16;64(Pt 5):m670-1. doi: 10.1107/S1600536808009835. Acta Crystallogr Sect E Struct Rep Online. 2008. PMID: 21202211 Free PMC article.
-
Chlorido{6,6'-dimethyl-2,2'-[1,2-phenyl-enebis(nitrilo-methyl-idyne)]diphenolato-κO,N,N',O'}manganese(III) mono-hydrate.Acta Crystallogr Sect E Struct Rep Online. 2008 Mar 12;64(Pt 4):m535-6. doi: 10.1107/S160053680800620X. Acta Crystallogr Sect E Struct Rep Online. 2008. PMID: 21201996 Free PMC article.
-
Chlorido{4,4'-dichloro-2,2'-[1,2-phenyl-enebis(nitrilo-methyl-idyne)]diphenolato-κO,N,N',O'}(methanol-κO)manganese(III).Acta Crystallogr Sect E Struct Rep Online. 2007 Dec 6;64(Pt 1):m124-5. doi: 10.1107/S1600536807064240. Acta Crystallogr Sect E Struct Rep Online. 2007. PMID: 21200483 Free PMC article.
-
The cocrystal aqua-chlorido{6,6'-di-tert-butyl-2,2'-[1,2-phenyl-enebis(nitrilo-methyl-idyne)]diphenolato-κO,N,N',O'}-manganese(III)-chlorido{6,6'-di-tert-butyl-2,2'-[1,2-phenyl-enebis(nitrilo-methyl-idyne)]diphenolato-κO,N,N',O'}(methanol-κO)manganese(III) (1/1).Acta Crystallogr Sect E Struct Rep Online. 2008 Apr 4;64(Pt 5):m626-7. doi: 10.1107/S1600536808006818. Acta Crystallogr Sect E Struct Rep Online. 2008. PMID: 21202181 Free PMC article.
-
Chlorido{4,4',6,6'-tetra-tert-butyl-2,2'-[o-phenyl-enebis(nitrilo-methyl-idyne)]diphenolato-κO,N,N',O'}manganese(III).Acta Crystallogr Sect E Struct Rep Online. 2009 Nov 28;65(Pt 12):m1692-3. doi: 10.1107/S1600536809050314. Acta Crystallogr Sect E Struct Rep Online. 2009. PMID: 21578697 Free PMC article.
Cited by
-
Chlorido{5,5'-dimeth-oxy-2,2'-[1,2-phenyl-enebis(nitrilo-methyl-idyne)]diphenolato-κO,N,N',O'}manganese(III).Acta Crystallogr Sect E Struct Rep Online. 2008 Apr 16;64(Pt 5):m670-1. doi: 10.1107/S1600536808009835. Acta Crystallogr Sect E Struct Rep Online. 2008. PMID: 21202211 Free PMC article.
-
General Approach to Silica-Supported Salens and Salophens and Their Use as Catalysts for the Synthesis of Cyclic Carbonates from Epoxides and Carbon Dioxide.J Org Chem. 2022 Dec 16;87(24):16410-16423. doi: 10.1021/acs.joc.2c02104. Epub 2022 Dec 1. J Org Chem. 2022. PMID: 36454692 Free PMC article.
-
Bis{μ-4,4'-dimeth-oxy-2,2'-[propane-1,2-diylbis(nitrilo-methyl-idyne)]diphenolato}bis-({4,4'-dimeth-oxy-2,2'-[propane-1,2-diylbis(nitrilo-methyl-idyne)]diphenol}manganese(III)) bis-(hexa-fluorido-phosphate).Acta Crystallogr Sect E Struct Rep Online. 2009 Jul 29;65(Pt 8):m1004-5. doi: 10.1107/S1600536809028591. Acta Crystallogr Sect E Struct Rep Online. 2009. PMID: 21583304 Free PMC article.
-
(E)-1-[(2-Amino-5-nitro-phen-yl)iminio-meth-yl]naphthalen-2-olate.Acta Crystallogr Sect E Struct Rep Online. 2010 Apr 30;66(Pt 5):o1227-8. doi: 10.1107/S1600536810014923. Acta Crystallogr Sect E Struct Rep Online. 2010. PMID: 21579252 Free PMC article.
-
4-Chloro-2-((E)-{3-[1-(hydroxy-imino)eth-yl]phen-yl}imino-meth-yl)phenol.Acta Crystallogr Sect E Struct Rep Online. 2009 Nov 7;65(Pt 12):o3021. doi: 10.1107/S1600536809045942. Acta Crystallogr Sect E Struct Rep Online. 2009. PMID: 21578758 Free PMC article.
References
-
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–S19.
-
- Bruker (2005). APEX2 (Version 1.27), SAINT (Version 7.12A) and SADABS (Version 2004/1). Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc 97, 1354–1358.
-
- Dixit, P. S. & Srinivasan, K. (1988). Inorg. Chem 27, 4507–4509.
LinkOut - more resources
Full Text Sources