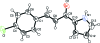
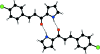
(2E)-3-(4-Chloro-phen-yl)-1-(1H-pyrrol-2-yl)prop-2-en-1-one
- PMID: 21202354
- PMCID: PMC2961151
- DOI: 10.1107/S1600536808010362
(2E)-3-(4-Chloro-phen-yl)-1-(1H-pyrrol-2-yl)prop-2-en-1-one
Abstract
In the mol-ecule of the title compound, C(13)H(10)ClNO, the benzene and pyrrole rings are oriented at a dihedral angle of 7.37 (12)°. In the crystal structure, inter-molecular N-H⋯O hydrogen bonds link the mol-ecules into centrosymmetric R(2) (2)(10) dimers. There are C-H⋯π inter-actions between benzene and pyrrole rings and a benzene C-H group. A weak π-π inter-action between the pyrrole rings [centroid-centroid distance 3.8515 (11) Å] further stabilizes the structure. There is also a π inter-action between the pyrrole ring and the carbonyl group, with a carbon-centroid distance of 3.4825 (18) Å.
Figures
References
-
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl.34, 1555–1573.
-
- Bruker (2005). SADABS Bruker AXS Inc. Madison, Wisconsion, USA.
-
- Bruker (2007). APEX2 and SAINT Bruker AXS Inc. Madison, Wisconsion, USA.
-
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
-
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous