3-Ammonio-pyridinium tetra-bromido-mercurate(II) monohydrate
- PMID: 21202445
- PMCID: PMC2961581
- DOI: 10.1107/S1600536808012336
3-Ammonio-pyridinium tetra-bromido-mercurate(II) monohydrate
Abstract
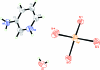
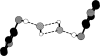
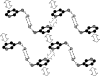
The asymmetric unit of the title compound, (C(5)H(8)N(2))[HgBr(4)]·H(2)O, consists of one cation, one anion and one water mol-ecule. The anion exhibits a distorted tetra-hedral arrangement about the Hg atom. The crystal structure contains alternating sheets of cations (in the ac plane) and stacks of anions. Several strong hydrogen-bonding inter-actions (pyN-H⋯Br and C-H⋯Br; py is pyridine), along with O-H⋯Br inter-actions, connect the sheets of cations to the stacks of anions. Cation-cation π-π stacking is also present (C⋯C distances in the range 3.424-3.865 Å). The shortest Br⋯Br distance is 3.9527 (9) Å.
Figures

References
-
- Al-Far, R. & Ali, B. F. (2007a). Acta Cryst. C63, m137–m139. - PubMed
-
- Al-Far, R. & Ali, B. F. (2007b). J. Chem. Crystallogr.37, 331–341.
-
- Al-Far, R., Ali, B. F. & Al-Sou’od, K. (2006). J. Chem. Crystallogr.36, 523–529.
-
- Ali, B. F. & Al-Far, R. (2007a). Acta Cryst. C63, m451–m453. - PubMed
-
- Ali, B. F. & Al-Far, R. (2007b). Acta Cryst. E63, m892–m894.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
