Poly[μ-aqua-diaqua-(μ(3)-N'-carboxy-methyl-ethylenediamine-N,N,N'-tri-acetato)oxidopotassium(I)vanadium(IV)]
- PMID: 21202763
- PMCID: PMC2961877
- DOI: 10.1107/S1600536808017030
Poly[μ-aqua-diaqua-(μ(3)-N'-carboxy-methyl-ethylenediamine-N,N,N'-tri-acetato)oxidopotassium(I)vanadium(IV)]
Abstract
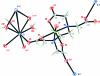
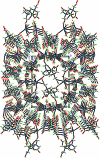
In the crystal structure of the title compound, [KV(C(10)H(13)N(2)O(8))O(H(2)O)(3)](n), the V(IV) ion adopts a distorted octa-hedral geometry, coordinated by one oxide group, two N and three carboxylate O atoms from the same N'-carboxy-methyl-ethyl-ene-diamine-N,N,N'-triacetate (HEDTA) ligand. The potassium ion is hepta-coordinated by two water mol-ecules, two bridging water mol-ecules and three carboxylate O atoms from three neighbouring HEDTA ligands. The HEDTA ligands and some of the water mol-ecules act as bridges, linking the compound into a three-dimensional architecture via 2(1) screw, c-glide, translation and inversion symmetry operators. Meanwhile, three types of O-H⋯O hydrogen bonds provide an additional stabilization of the three-dimensional architecture.
Figures
References
-
- Bruker (2000). SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Crans, D. C., Smee, J. J., Gaidamauskas, E. & Yang, L. (2004). Chem. Rev.104, 849–902. - PubMed
-
- Khanra, S., Kloth, M., Mansaray, H., Muryn, C. A., Tuna, F., Sanudo, E. C., Helliwell, M., McInnes, E. J. L. & Winpenny, R. E. P. (2007). Angew. Chem. Int. Ed.46, 5568–5571. - PubMed
-
- Sheldrick, G. M. (2000). SADABS University of Göttingen, Germany.
-
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials