Bis(μ-N-benzyl-N-methyl-dithio-carbamato)-1:2κS,S':S';1:2κS:S,S'-bis-[bis-(N-benzyl-N-methyl-dithio-carbamato-κS,S')thallium(III)]
- PMID: 21203013
- PMCID: PMC2961943
- DOI: 10.1107/S1600536808021004
Bis(μ-N-benzyl-N-methyl-dithio-carbamato)-1:2κS,S':S';1:2κS:S,S'-bis-[bis-(N-benzyl-N-methyl-dithio-carbamato-κS,S')thallium(III)]
Abstract
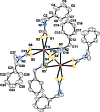
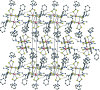
The molecule of the dinuclear title compound, [Tl(2)(C(9)H(10)NS(2))(6)], possesses a crystallographically imposed centre of symmetry. Each Tl(III) atom is seven-coordinated by S atoms of four different dithio-carbamate anions in a distorted penta-gonal-bipyramidal coordination geometry. The crystal structure is stabilized by a C-H⋯S hydrogen-bond inter-action linking complex mol-ecules into chains running parallel to the b axis. Intramolecular C-H⋯S hydrogen bonds are also present.
Figures
References
-
- Abrahamson, H., Heiman, J. R. & Pignolet, L. H. (1975). Inorg. Chem.14, 2070–2075.
-
- Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst.32, 115–119.
-
- Bruker (1997). SMART, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Burschka, C. H. (1982). Z. Anorg. Allg. Chem.485, 217–224.
-
- Casas, J. S., Castaño, M. V., Freire, C., Sanchez, A., Sordo, J., Castellano, E. E. & Zukerman-Schpector, J. (1994). Inorg. Chim. Acta, 216, 15–20.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
