(E)-1,2-Bis(4-fluoro-phen-yl)ethane-1,2-dione
- PMID: 21203308
- PMCID: PMC2962135
- DOI: 10.1107/S1600536808023350
(E)-1,2-Bis(4-fluoro-phen-yl)ethane-1,2-dione
Abstract
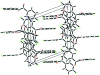
The title compound, C(14)H(8)F(2)O(2), is a substituted benzil with an s-trans conformation of the dicarbonyl unit. This conformation is also shown by the O-C-C-O torsion angle of -110.65 (12)°. An unusual feature of the structure is the length, 1.536 (2) Å, of the central C-C bond connecting the carbonyl units, which is significantly longer than a normal Csp(2)-Csp(2) single bond. This is probably the result of decreasing the unfavourable vicinal dipole-dipole inter-actions by increasing the distance between the two electronegative O atoms [O⋯O = 3.1867 (12) Å] and allowing orbital overlap of the dione with the π system of the benzene rings. The dihedral angle between the aromatic rings is 64.74 (5)°. In the crystal structure, neighbouring mol-ecules are linked together by weak inter-molecular C-H⋯O (× 2) hydrogen bonds. In addition, the crystal structure is further stabilized by inter-molecular π-π inter-actions with centroid-centroid distances in the range 3.6416 (6)-3.7150 (7) Å.
Figures
References
-
- Allen, F. H., Baalham, C. A., Lommerse, J. P. M. & Raithby, P. R. (1998). Acta Cryst. B54, 320–329.
-
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–S19.
-
- Brown, C. J. & Sadanaga, R. (1965). Acta Cryst.18, 158–164.
-
- Bruker (2005). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Crowley, J. I., Balanson, R. D. & Mayerle, J. J. (1983). J. Am. Chem. Soc. 105, 6416–6422.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous